当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel Au Catalysis Strategy for the Synthesis of Au@Pt Core–Shell Nanoelectrocatalyst with Self-Controlled Quasi-Monolayer Pt Skin
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acsami.7b08210 Youlin Zhang 1 , Xiaokun Li , Kai Li , Bin Xue 1 , Chunmei Zhang , Cheng Du , Zhijian Wu , Wei Chen
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acsami.7b08210 Youlin Zhang 1 , Xiaokun Li , Kai Li , Bin Xue 1 , Chunmei Zhang , Cheng Du , Zhijian Wu , Wei Chen
Affiliation
|
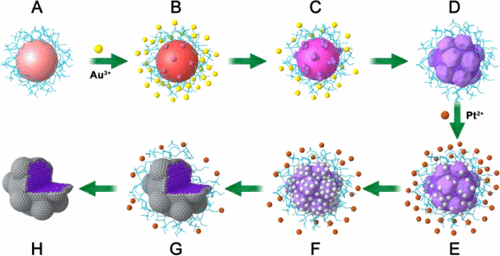
Design of catalytically active Pt-based catalysts with minimizing the usage of Pt is a major issue in fuel cells. Herein, for the first time, we have developed a Au catalytic reduction strategy to synthesize a Au@Pt core–shell electrocatalyst with a quasi-monolayer Pt skin spontaneously formed from the gold surface catalysis. In the presence of presynthesized gold nanocrystals (used as the catalyst and Au seeds) and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer (used as reductant), under the Au surface catalysis, platinum ions can be reduced and deposited on the gold nanocrystals to form a Pt skin surface with a quasi-monatomic thickness. In the present strategy, Pt ions can be reduced only under the catalysis of gold surface and thus when the surface of Au NPs is covered by a monatomic Pt layer, the reduction reaction of Pt ions will be spontaneously turned off. Therefore, the significant advantage of this synthesis strategy is that the formation of quasi-monolayer Pt skin surface can be self-controlled and is completely free of controlling the dosage of platinum ions and the size distribution of Au cores. The synthesized Au@Pt core@shell structure exhibited enhanced electrocatalytic activities for oxygen reduction reaction and methanol oxidation reaction, which are 6.87 and 10.17 times greater than those of Pt/C catalyst, respectively. The present study provides a new strategy for obtaining high-performance bimetallic/multimetallic electrocatalysts with high utilization of precious metals.
中文翻译:

具有自控准单层铂皮的Au @ Pt核壳纳米电催化剂的合成新Au催化策略
在燃料电池中,设计具有最小化Pt使用量的具有催化活性的Pt基催化剂是一个主要问题。在此,我们首次开发了一种Au催化还原策略,用于合成Au @ Pt核壳电催化剂和由金表面催化自发形成的准单层Pt皮。在预先合成的金纳米晶体(用作催化剂和Au种子)和4-(2-羟乙基)-1-哌嗪乙烷磺酸磺酸缓冲液(用作还原剂)的存在下,在Au表面催化下,铂离子可被还原并沉积在金纳米晶体形成具有准单原子厚度的Pt皮肤表面。在目前的策略中,仅在金表面催化下才能还原Pt离子,因此当Au NPs的表面被单原子Pt层覆盖时,Pt离子的还原反应将自发关闭。因此,该合成策略的显着优点是准单层Pt皮肤表面的形成可以自我控制,并且完全无需控制铂离子的剂量和Au核的尺寸分布。合成的Au @ Pt核壳结构对氧还原反应和甲醇氧化反应具有增强的电催化活性,分别是Pt / C催化剂的6.87倍和10.17倍。本研究为获得高利用率的贵金属双金属/多金属高性能电催化剂提供了一种新的策略。该合成策略的显着优势在于,准单层Pt皮肤表面的形成可以自我控制,并且完全无需控制铂离子的剂量和Au核的尺寸分布。合成的Au @ Pt核壳结构对氧还原反应和甲醇氧化反应具有增强的电催化活性,分别是Pt / C催化剂的6.87倍和10.17倍。本研究为获得高利用率的贵金属双金属/多金属高性能电催化剂提供了一种新的策略。该合成策略的显着优势在于,准单层Pt皮肤表面的形成可以自我控制,并且完全无需控制铂离子的剂量和Au核的尺寸分布。合成的Au @ Pt核壳结构对氧还原反应和甲醇氧化反应具有增强的电催化活性,分别是Pt / C催化剂的6.87倍和10.17倍。本研究为获得高利用率的贵金属双金属/多金属高性能电催化剂提供了一种新的策略。合成的Au @ Pt核壳结构对氧还原反应和甲醇氧化反应具有增强的电催化活性,分别是Pt / C催化剂的6.87倍和10.17倍。本研究为获得高利用率的贵金属双金属/多金属高性能电催化剂提供了一种新的策略。合成的Au @ Pt核壳结构对氧还原反应和甲醇氧化反应具有增强的电催化活性,分别是Pt / C催化剂的6.87倍和10.17倍。本研究为获得高利用率的贵金属双金属/多金属高性能电催化剂提供了一种新的策略。
更新日期:2017-09-15
中文翻译:

具有自控准单层铂皮的Au @ Pt核壳纳米电催化剂的合成新Au催化策略
在燃料电池中,设计具有最小化Pt使用量的具有催化活性的Pt基催化剂是一个主要问题。在此,我们首次开发了一种Au催化还原策略,用于合成Au @ Pt核壳电催化剂和由金表面催化自发形成的准单层Pt皮。在预先合成的金纳米晶体(用作催化剂和Au种子)和4-(2-羟乙基)-1-哌嗪乙烷磺酸磺酸缓冲液(用作还原剂)的存在下,在Au表面催化下,铂离子可被还原并沉积在金纳米晶体形成具有准单原子厚度的Pt皮肤表面。在目前的策略中,仅在金表面催化下才能还原Pt离子,因此当Au NPs的表面被单原子Pt层覆盖时,Pt离子的还原反应将自发关闭。因此,该合成策略的显着优点是准单层Pt皮肤表面的形成可以自我控制,并且完全无需控制铂离子的剂量和Au核的尺寸分布。合成的Au @ Pt核壳结构对氧还原反应和甲醇氧化反应具有增强的电催化活性,分别是Pt / C催化剂的6.87倍和10.17倍。本研究为获得高利用率的贵金属双金属/多金属高性能电催化剂提供了一种新的策略。该合成策略的显着优势在于,准单层Pt皮肤表面的形成可以自我控制,并且完全无需控制铂离子的剂量和Au核的尺寸分布。合成的Au @ Pt核壳结构对氧还原反应和甲醇氧化反应具有增强的电催化活性,分别是Pt / C催化剂的6.87倍和10.17倍。本研究为获得高利用率的贵金属双金属/多金属高性能电催化剂提供了一种新的策略。该合成策略的显着优势在于,准单层Pt皮肤表面的形成可以自我控制,并且完全无需控制铂离子的剂量和Au核的尺寸分布。合成的Au @ Pt核壳结构对氧还原反应和甲醇氧化反应具有增强的电催化活性,分别是Pt / C催化剂的6.87倍和10.17倍。本研究为获得高利用率的贵金属双金属/多金属高性能电催化剂提供了一种新的策略。合成的Au @ Pt核壳结构对氧还原反应和甲醇氧化反应具有增强的电催化活性,分别是Pt / C催化剂的6.87倍和10.17倍。本研究为获得高利用率的贵金属双金属/多金属高性能电催化剂提供了一种新的策略。合成的Au @ Pt核壳结构对氧还原反应和甲醇氧化反应具有增强的电催化活性,分别是Pt / C催化剂的6.87倍和10.17倍。本研究为获得高利用率的贵金属双金属/多金属高性能电催化剂提供了一种新的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号