当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Confined Catalysis in the g-C3N4/Pt(111) Interface: Feasible Molecule Intercalation, Tunable Molecule–Metal Interaction, and Enhanced Reaction Activity of CO Oxidation
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acsami.7b08665 Shujiao Wang 1 , Yingxin Feng 1 , Ming’an Yu 1 , Qiang Wan 1 , Sen Lin 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acsami.7b08665 Shujiao Wang 1 , Yingxin Feng 1 , Ming’an Yu 1 , Qiang Wan 1 , Sen Lin 1
Affiliation
|
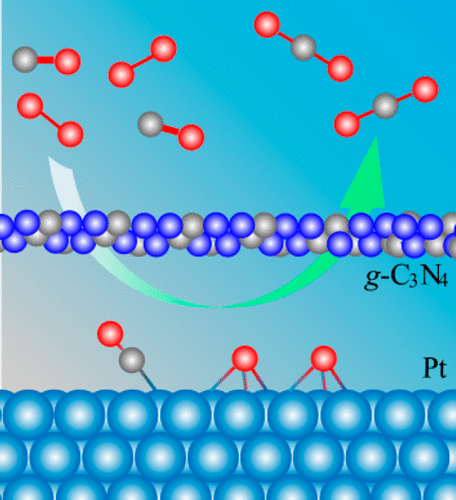
The deposition of a two-dimensional (2D) atomic nanosheet on a metal surface has been considered as a new route for tuning the molecule–metal interaction and surface reactivity in terms of the confinement effect. In this work, we use first-principles calculations to systematically explore a novel nanospace constructed by placing a 2D graphitic carbon nitride (g-C3N4) nanosheet over a Pt(111) surface. The confined catalytic activity in this nanospace is investigated using CO oxidation as a model reaction. With the inherent triangular pores in the g-C3N4 overlayer being taken advantage of, molecules such as CO and O2 can diffuse to adsorb on the Pt(111) surface underneath the g-C3N4 overlayer. Moreover, the mechanism of intercalation is also elucidated, and the results reveal that the energy barrier depends mainly on the properties of the molecule and the channel. Importantly, the molecule–catalyst interaction can be tuned by the g-C3N4 overlayer, considerably reducing the adsorption energy of CO on Pt(111) and leading to enhanced reactivity in CO oxidation. This work will provide important insight for constructing a promising nanoreactor in which the following is observed: The molecule intercalation is facile; the molecule–metal interaction is efficiently tuned; the metal-catalyzed reaction is promoted.
中文翻译:

g -C 3 N 4 / Pt(111)界面中的受限催化:可行的分子嵌入,可调的分子-金属相互作用和增强的CO氧化反应活性
二维(2D)原子纳米片在金属表面上的沉积被认为是根据限制效应调整分子-金属相互作用和表面反应性的新途径。在这项工作中,我们使用第一原理计算系统地探索通过在Pt(111)表面上放置2D石墨氮化碳(g -C 3 N 4)纳米片而构造的新型纳米空间。使用CO氧化作为模型反应,研究了该纳米空间中的受限催化活性。利用g -C 3 N 4覆盖层中固有的三角形孔,可利用诸如CO和O 2之类的分子可以扩散到吸附在g -C 3 N 4覆盖层下面的Pt(111)表面上。此外,还阐明了嵌入的机理,结果表明能垒主要取决于分子和通道的性质。重要的是,可以通过g -C 3 N 4来调节分子与催化剂的相互作用。覆盖层,大大降低了CO在Pt(111)上的吸附能,并提高了CO氧化的反应性。这项工作将为构建有前景的纳米反应器提供重要的见识,其中观察到以下情况:分子插入容易;有效地调节了分子与金属的相互作用;促进了金属催化的反应。
更新日期:2017-09-15
中文翻译:

g -C 3 N 4 / Pt(111)界面中的受限催化:可行的分子嵌入,可调的分子-金属相互作用和增强的CO氧化反应活性
二维(2D)原子纳米片在金属表面上的沉积被认为是根据限制效应调整分子-金属相互作用和表面反应性的新途径。在这项工作中,我们使用第一原理计算系统地探索通过在Pt(111)表面上放置2D石墨氮化碳(g -C 3 N 4)纳米片而构造的新型纳米空间。使用CO氧化作为模型反应,研究了该纳米空间中的受限催化活性。利用g -C 3 N 4覆盖层中固有的三角形孔,可利用诸如CO和O 2之类的分子可以扩散到吸附在g -C 3 N 4覆盖层下面的Pt(111)表面上。此外,还阐明了嵌入的机理,结果表明能垒主要取决于分子和通道的性质。重要的是,可以通过g -C 3 N 4来调节分子与催化剂的相互作用。覆盖层,大大降低了CO在Pt(111)上的吸附能,并提高了CO氧化的反应性。这项工作将为构建有前景的纳米反应器提供重要的见识,其中观察到以下情况:分子插入容易;有效地调节了分子与金属的相互作用;促进了金属催化的反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号