当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Nitrogenase H2 Formation by Metal-Hydride Protonation Probed by Mediated Electrocatalysis and H/D Isotope Effects
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/jacs.7b07311 Nimesh Khadka 1 , Ross D. Milton 2 , Sudipta Shaw 1 , Dmitriy Lukoyanov 3 , Dennis R. Dean 4 , Shelley D. Minteer 2 , Simone Raugei 5 , Brian M. Hoffman 3 , Lance C. Seefeldt 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/jacs.7b07311 Nimesh Khadka 1 , Ross D. Milton 2 , Sudipta Shaw 1 , Dmitriy Lukoyanov 3 , Dennis R. Dean 4 , Shelley D. Minteer 2 , Simone Raugei 5 , Brian M. Hoffman 3 , Lance C. Seefeldt 1
Affiliation
|
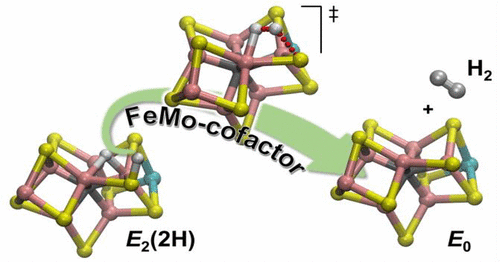
Nitrogenase catalyzes the reduction of dinitrogen (N2) to two ammonia (NH3) at its active site FeMo-cofactor through a mechanism involving reductive elimination of two [Fe–H–Fe] bridging hydrides to make H2. A competing reaction is the protonation of the hydride [Fe–H–Fe] to make H2. The overall nitrogenase rate-limiting step is associated with ATP-driven electron delivery from Fe protein, precluding isotope effect measurements on substrate reduction steps. Here, we use mediated bioelectrocatalysis to drive electron delivery to the MoFe protein allowing examination of the mechanism of H2 formation by the metal-hydride protonation reaction. The ratio of catalytic current in mixtures of H2O and D2O, the proton inventory, was found to change linearly with the D2O/H2O ratio, revealing that a single H/D is involved in the rate-limiting step of H2 formation. Kinetic models, along with measurements that vary the electron/proton delivery rate and use different substrates, reveal that the rate-limiting step under these conditions is the H2 formation reaction. Altering the chemical environment around the active site FeMo-cofactor in the MoFe protein, either by substituting nearby amino acids or transferring the isolated FeMo-cofactor into a different peptide matrix, changes the net isotope effect, but the proton inventory plot remains linear, consistent with an unchanging rate-limiting step. Density functional theory predicts a transition state for H2 formation where the S–H+ bond breaks and H+ attacks the Fe-hydride, and explains the observed H/D isotope effect. This study not only reveals the nitrogenase mechanism of H2 formation by hydride protonation, but also illustrates a strategy for mechanistic study that can be applied to other oxidoreductase enzymes and to biomimetic complexes.
中文翻译:

介导的电催化和H / D同位素效应探测金属氢化物质子化形成固氮酶H 2的机理
固氮酶通过一种涉及还原性消除两个[Fe–H–Fe]桥接氢化物以生成H 2的机制,在其活性位点FeMo-辅因子上将二氮(N 2)还原为两个氨(NH 3)。竞争的反应是氢化物[Fe–H–Fe]的质子化,生成H 2。总体固氮酶限速步骤与铁蛋白从ATP驱动的电子传递有关,从而排除了对底物还原步骤进行同位素效应的测量。在这里,我们使用介导的生物电催化来驱动电子传递到MoFe蛋白,从而允许通过金属氢化物质子化反应检查H 2形成的机理。H 2混合物中的催化电流比发现O和D 2 O(质子存量)随D 2 O / H 2 O之比线性变化,表明单个H / D参与H 2形成的限速步骤。动力学模型以及改变电子/质子传递速率并使用不同底物的测量结果表明,在这些条件下的限速步骤是H 2形成反应。通过取代附近的氨基酸或将分离的FeMo辅因子转移到不同的肽基质中来改变MoFe蛋白质中活性位点FeMo辅因子周围的化学环境,会改变净同位素效应,但质子库图仍保持线性一致具有不变的速率限制步骤。密度泛函理论预测了H 2形成的过渡态,其中S–H +键断裂而H +攻击氢化铁,并解释了观察到的H / D同位素效应。这项研究不仅揭示了H 2的固氮酶机制 氢化物质子化的形成,但也说明了可用于其他氧化还原酶和仿生复合物的机理研究策略。
更新日期:2017-09-15
中文翻译:

介导的电催化和H / D同位素效应探测金属氢化物质子化形成固氮酶H 2的机理
固氮酶通过一种涉及还原性消除两个[Fe–H–Fe]桥接氢化物以生成H 2的机制,在其活性位点FeMo-辅因子上将二氮(N 2)还原为两个氨(NH 3)。竞争的反应是氢化物[Fe–H–Fe]的质子化,生成H 2。总体固氮酶限速步骤与铁蛋白从ATP驱动的电子传递有关,从而排除了对底物还原步骤进行同位素效应的测量。在这里,我们使用介导的生物电催化来驱动电子传递到MoFe蛋白,从而允许通过金属氢化物质子化反应检查H 2形成的机理。H 2混合物中的催化电流比发现O和D 2 O(质子存量)随D 2 O / H 2 O之比线性变化,表明单个H / D参与H 2形成的限速步骤。动力学模型以及改变电子/质子传递速率并使用不同底物的测量结果表明,在这些条件下的限速步骤是H 2形成反应。通过取代附近的氨基酸或将分离的FeMo辅因子转移到不同的肽基质中来改变MoFe蛋白质中活性位点FeMo辅因子周围的化学环境,会改变净同位素效应,但质子库图仍保持线性一致具有不变的速率限制步骤。密度泛函理论预测了H 2形成的过渡态,其中S–H +键断裂而H +攻击氢化铁,并解释了观察到的H / D同位素效应。这项研究不仅揭示了H 2的固氮酶机制 氢化物质子化的形成,但也说明了可用于其他氧化还原酶和仿生复合物的机理研究策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号