当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Functionalized Tertiary-Alkylative Sonogashira Type Couplings via Copper Acetylide at Room Temperature
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acscatal.7b02615 Yu Yamane 1 , Naoki Miwa 1 , Takashi Nishikata 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acscatal.7b02615 Yu Yamane 1 , Naoki Miwa 1 , Takashi Nishikata 1
Affiliation
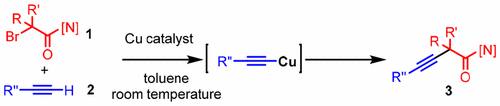
|
There are several reports on Sonogashira couplings, but most of the reported reactions have employed aryl or alkenyl halides as coupling partners. Therefore, Sonogashira coupling is unsuitable for alkyl loadings, especially tertiary alkyl groups. In this research, we found that a copper catalyst is effective for a reaction between a terminal alkyne and an α-bromocarbonyl compound to form a quaternary carbon having alkynyl group at room temperature. Control experiments revealed that a copper acetylide is a key intermediate.
中文翻译:

室温下乙炔铜对铜催化的功能化叔烷基Sonogashira型偶联
关于Sonogashira偶联的报道很多,但是大多数报道的反应都使用了芳基或烯基卤化物作为偶联伴侣。因此,Sonogashira偶联不适合烷基负载,尤其是叔烷基。在该研究中,我们发现铜催化剂对于末端炔与α-溴代羰基化合物在室温下形成具有炔基的季碳的反应是有效的。对照实验表明,乙炔铜是关键中间体。
更新日期:2017-09-15
中文翻译:

室温下乙炔铜对铜催化的功能化叔烷基Sonogashira型偶联
关于Sonogashira偶联的报道很多,但是大多数报道的反应都使用了芳基或烯基卤化物作为偶联伴侣。因此,Sonogashira偶联不适合烷基负载,尤其是叔烷基。在该研究中,我们发现铜催化剂对于末端炔与α-溴代羰基化合物在室温下形成具有炔基的季碳的反应是有效的。对照实验表明,乙炔铜是关键中间体。










































 京公网安备 11010802027423号
京公网安备 11010802027423号