当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient route for the construction of polycyclic systems from bioderived HMF
Green Chemistry ( IF 9.3 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1039/c7gc02211e F. A. Kucherov 1, 2, 3, 4 , K. I. Galkin 1, 2, 3, 4 , E. G. Gordeev 1, 2, 3, 4 , V. P. Ananikov 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1039/c7gc02211e F. A. Kucherov 1, 2, 3, 4 , K. I. Galkin 1, 2, 3, 4 , E. G. Gordeev 1, 2, 3, 4 , V. P. Ananikov 1, 2, 3, 4
Affiliation
|
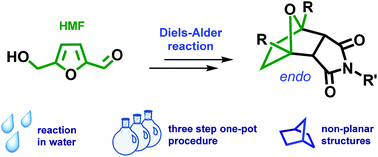
The first synthesis of tricyclic compounds from biobased 5-hydroxymethylfurfural (HMF) is described. The Diels–Alder reaction was used to implement the transition from HMF to a non-planar framework, which possessed structural cores of naturally occurring biologically active compounds and building blocks of advanced materials. A one-pot, three-step sustainable synthesis in water was developed starting directly from HMF. The reduction of HMF led to 2,5-bis(hydroxymethyl)furan (BHMF), which could be readily involved in the Diels–Alder cycloaddition reaction with HMF-derived maleimide, followed by hydrogenation of the double bond. The described transformation was diastereoselective and proceeded with a good overall yield. The applicability of the chosen approach for the synthesis of analogous structures containing amine functionality on the side chain was demonstrated. To produce the target compounds, only platform chemicals were used with carbohydrate biomass as the single carbon source.
中文翻译:

由生物来源的HMF构建多环系统的有效途径
描述了从生物基5-羟甲基糠醛(HMF)首次合成三环化合物。Diels-Alder反应用于实现从HMF到非平面框架的过渡,该框架具有天然存在的生物活性化合物的结构核心和先进材料的构造块。直接从HMF开始开发了一个一锅,三步,可持续的水中合成方法。HMF的还原产生了2,5-双(羟甲基)呋喃(BHMF),它很容易与HMF衍生的马来酰亚胺进行Diels-Alder环加成反应,然后双键氢化。所描述的转化是非对映选择性的,并以良好的总产率进行。证明了所选择的方法在侧链上含有胺官能团的类似结构的合成中的适用性。为了生产目标化合物,仅将平台化学品与碳水化合物生物质作为单一碳源一起使用。
更新日期:2017-09-15
中文翻译:

由生物来源的HMF构建多环系统的有效途径
描述了从生物基5-羟甲基糠醛(HMF)首次合成三环化合物。Diels-Alder反应用于实现从HMF到非平面框架的过渡,该框架具有天然存在的生物活性化合物的结构核心和先进材料的构造块。直接从HMF开始开发了一个一锅,三步,可持续的水中合成方法。HMF的还原产生了2,5-双(羟甲基)呋喃(BHMF),它很容易与HMF衍生的马来酰亚胺进行Diels-Alder环加成反应,然后双键氢化。所描述的转化是非对映选择性的,并以良好的总产率进行。证明了所选择的方法在侧链上含有胺官能团的类似结构的合成中的适用性。为了生产目标化合物,仅将平台化学品与碳水化合物生物质作为单一碳源一起使用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号