当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-oxidative dehydrogenation of propane, n-butane, and isobutane over bulk ZrO2-based catalysts: effect of dopant on the active site and pathways of product formation
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1039/c7cy01583f Tatiana P. Otroshchenko 1, 2, 3 , Vita A. Kondratenko 1, 2, 3 , Uwe Rodemerck 1, 2, 3 , David Linke 1, 2, 3 , Evgenii V. Kondratenko 1, 2, 3
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1039/c7cy01583f Tatiana P. Otroshchenko 1, 2, 3 , Vita A. Kondratenko 1, 2, 3 , Uwe Rodemerck 1, 2, 3 , David Linke 1, 2, 3 , Evgenii V. Kondratenko 1, 2, 3
Affiliation
|
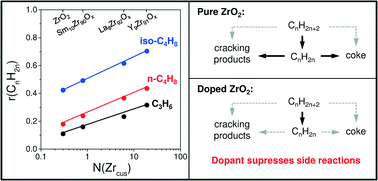
Non-oxidative dehydrogenation (DH) of propane, n-butane, and isobutane was investigated over bare ZrO2 and binary MZrOx (M = Li, Ca, Mg, Y, Sm or La) materials. Selected samples were characterized by XRD, Raman spectroscopy, NH3 temperature-programmed desorption, IR spectroscopy and O2 titration experiments. A correlation between the rate of olefin formation and the concentration of anion vacancies formed upon reductive catalyst treatment was established. Such defects represent the catalytically active sites, which are coordinatively unsaturated Zr4+ (Zrcus) cations. From a structural viewpoint, the active Zrcus sites should have a lower coordination number than the Zrcus sites located on the top of an ideal flat surface, thus highlighting the importance of surface defects in the target reactions. However, the intrinsic activity of such sites and/or their concentration depends on the kind of dopant for ZrO2. The dopant was also shown to inhibit side reactions especially those leading to coke due to reducing the concentration of strong acidic sites related to ZrO2. Similar to an analogue of industrial K-CrOx/Al2O3 catalyst, all binary catalysts exhibited low activity for consecutive reactions of the target olefins; the desired selectivity was higher than 85% at an alkane conversion of about 30%. With respect to the feed alkane, primary selectivity (at a zero degree of alkane conversion) to the target olefin decreases in the order: C3H8, iso-C4H10, and n-C4H10. This was explained by the higher stability of surface intermediates formed from the latter two contributing to the formation of coke and 1,3-butadiene, respectively.
中文翻译:

基于本体ZrO 2的催化剂上丙烷,正丁烷和异丁烷的非氧化脱氢:掺杂物对活性位点和产物形成途径的影响
在裸露的ZrO 2和二元MZrO x(M = Li,Ca,Mg,Y,Sm或La)材料上研究了丙烷,正丁烷和异丁烷的非氧化脱氢(DH)。通过XRD,拉曼光谱,NH 3程序升温脱附,IR光谱和O 2滴定实验对选定的样品进行表征。建立了烯烃形成速率与还原催化剂处理后形成的阴离子空位浓度之间的相关性。这些缺陷代表催化活性位点,它们是配位不饱和的Zr 4+(Zr cus)阳离子。从结构的角度来看,活性Zr cus与理想平整表面顶部的Zr cus位点相比,这些位点应具有较低的配位数,因此突出了目标反应中表面缺陷的重要性。然而,这些位点的固有活性和/或它们的浓度取决于ZrO 2的掺杂剂种类。由于降低了与ZrO 2有关的强酸性部位的浓度,该掺杂剂还显示出抑制副反应的作用,特别是那些导致焦炭的副反应。类似于工业K-CrO x / Al 2 O 3的类似物催化剂,所有二元催化剂对目标烯烃的连续反应均表现出较低的活性;在约30%的烷烃转化率下,所需的选择性高于85%。关于进料烷烃,对目标烯烃的初级选择性(在烷烃转化度为零时)依次降低:C 3 H 8,异-C 4 H 10和nC 4 H 10。由后两种形成的表面中间体的较高稳定性可以解释,后者分别有助于形成焦炭和1,3-丁二烯。
更新日期:2017-09-15
中文翻译:

基于本体ZrO 2的催化剂上丙烷,正丁烷和异丁烷的非氧化脱氢:掺杂物对活性位点和产物形成途径的影响
在裸露的ZrO 2和二元MZrO x(M = Li,Ca,Mg,Y,Sm或La)材料上研究了丙烷,正丁烷和异丁烷的非氧化脱氢(DH)。通过XRD,拉曼光谱,NH 3程序升温脱附,IR光谱和O 2滴定实验对选定的样品进行表征。建立了烯烃形成速率与还原催化剂处理后形成的阴离子空位浓度之间的相关性。这些缺陷代表催化活性位点,它们是配位不饱和的Zr 4+(Zr cus)阳离子。从结构的角度来看,活性Zr cus与理想平整表面顶部的Zr cus位点相比,这些位点应具有较低的配位数,因此突出了目标反应中表面缺陷的重要性。然而,这些位点的固有活性和/或它们的浓度取决于ZrO 2的掺杂剂种类。由于降低了与ZrO 2有关的强酸性部位的浓度,该掺杂剂还显示出抑制副反应的作用,特别是那些导致焦炭的副反应。类似于工业K-CrO x / Al 2 O 3的类似物催化剂,所有二元催化剂对目标烯烃的连续反应均表现出较低的活性;在约30%的烷烃转化率下,所需的选择性高于85%。关于进料烷烃,对目标烯烃的初级选择性(在烷烃转化度为零时)依次降低:C 3 H 8,异-C 4 H 10和nC 4 H 10。由后两种形成的表面中间体的较高稳定性可以解释,后者分别有助于形成焦炭和1,3-丁二烯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号