当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The photochemical alkylation and reduction of heteroarenes
Chemical Science ( IF 7.6 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1039/c7sc03768f T. McCallum 1, 2, 3, 4, 5 , S. P. Pitre 1, 2, 3, 4, 5 , M. Morin 1, 2, 3, 4, 5 , J. C. Scaiano 1, 2, 3, 4, 5 , L. Barriault 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1039/c7sc03768f T. McCallum 1, 2, 3, 4, 5 , S. P. Pitre 1, 2, 3, 4, 5 , M. Morin 1, 2, 3, 4, 5 , J. C. Scaiano 1, 2, 3, 4, 5 , L. Barriault 1, 2, 3, 4, 5
Affiliation
|
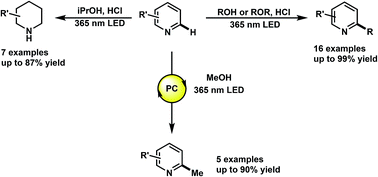
The functionalization of heteroarenes has been integral to the structural diversification of medicinally active molecules such as quinolines, pyridines, and phenanthridines. Electron-deficient heteroarenes are electronically compatible to react with relatively nucleophilic free radicals such as hydroxyalkyl. However, the radical functionalization of such heteroarenes has been marked by the use of transition-metal catalyzed processes that require initiators and stoichiometric oxidants. Herein, we describe the photochemical alkylation of quinolines, pyridines and phenanthridines, where through direct excitation of the protonated heterocycle, alcohols and ethers, such as methanol and THF, can serve as alkylating agents. We also report the discovery of a photochemical reduction of these heteroarenes using only iPrOH and HCl. Mechanistic studies to elucidate the underlying mechanism of these transformations, and preliminary results on catalytic methylations are also reported.
中文翻译:

光化学烷基化和杂芳烃的还原
杂芳烃的功能化对于诸如喹啉,吡啶和菲啶之类的医学活性分子的结构多样化是不可或缺的。缺电子的杂芳烃具有电子相容性,可与相对亲核的自由基(例如羟烷基)反应。然而,这种杂芳烃的自由基官能化已通过使用过渡金属催化的方法来标记,该方法需要引发剂和化学计量的氧化剂。本文中,我们描述了喹啉,吡啶和菲啶的光化学烷基化,其中通过直接激发质子化的杂环,醇和醚(例如甲醇和THF)可以用作烷基化剂。我们还报告了仅使用iPrOH和HCl光化学还原这些杂芳烃的发现。
更新日期:2017-09-15
中文翻译:

光化学烷基化和杂芳烃的还原
杂芳烃的功能化对于诸如喹啉,吡啶和菲啶之类的医学活性分子的结构多样化是不可或缺的。缺电子的杂芳烃具有电子相容性,可与相对亲核的自由基(例如羟烷基)反应。然而,这种杂芳烃的自由基官能化已通过使用过渡金属催化的方法来标记,该方法需要引发剂和化学计量的氧化剂。本文中,我们描述了喹啉,吡啶和菲啶的光化学烷基化,其中通过直接激发质子化的杂环,醇和醚(例如甲醇和THF)可以用作烷基化剂。我们还报告了仅使用iPrOH和HCl光化学还原这些杂芳烃的发现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号