当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into Concerted C–C Reductive Elimination from Homoleptic Uranium Alkyls
Organometallics ( IF 2.5 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acs.organomet.7b00438 Sara A. Johnson 1 , Robert F. Higgins 2 , Mahdi M. Abu-Omar 1 , Matthew P. Shores 2 , Suzanne C. Bart 1
Organometallics ( IF 2.5 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acs.organomet.7b00438 Sara A. Johnson 1 , Robert F. Higgins 2 , Mahdi M. Abu-Omar 1 , Matthew P. Shores 2 , Suzanne C. Bart 1
Affiliation
|
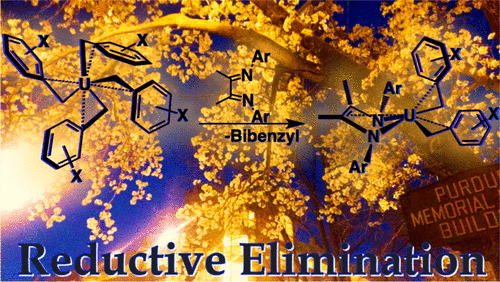
A mechanistic study was carried out to probe concerted C–C reductive elimination from homoleptic uranium(IV) alkyls. The para-chloro uranium(IV) tetrabenzyl derivative, U(CH2–p-ClC6H4)4 (2-p-Cl), was synthesized by treating UCl4 with 4 equivalents of KCH2–p-Cl-Ph (1-p-Cl) at −108 °C, adding a new member to the previously reported family of uranium alkyl complexes U(CH2C6H5)4 (2-Bn), U(CH2–p-iPrC6H4)4 (2-p-iPr), U(CH2–ptBu-C6H4)4 (2-p-tBu), U(CH2–o-OMeC6H4)4 (2-o-OMe), and U(CH2–m-OMeC6H4)4 (2-m-OMe). Each member of this family readily reacts with the redox-active α-diimine ligand, MesDABMe (MesDABMe = [MesN═C(Me)C(Me)═NMes]; Mes = 2,4,6-trimethylphenyl), to afford the products from C–C reductive elimination, namely, (MesDABMe)U(CH2Ph′)2 and Ph′CH2CH2Ph′ (Ph′ = p-iPrC6H4, p-tBuC6H4, m-OMeC6H4, p-ClC6H4). Room-temperature magnetic-susceptibility values, obtained via SQUID magnetometry, show a correlation with an increase in the magnetic moment as the electron-withdrawing character of the substituent increases. Kinetic studies were used to assess the effect of the benzyl substituent on the rate of reductive elimination, showing that reaction rate increases as the electron-withdrawing nature of the substitution increases. Eyring data revealed a large and negative entropy value, indicative of a highly ordered transition state, consistent with the previously reported concerted elimination concluded from crossover experiments.
中文翻译:

均相铀烷基的协同CC消除反应的机理研究
进行了一项机械研究,以探讨从均相铀(IV)烷基中协同进行的CC还原消除。的对位-氯铀(IV)四苄衍生物,U(CH 2 - p -ClC 6 ħ 4)4(2- p -Cl),通过处理UCL合成4与4个当量的KCH 2 - p -Cl-PH (1-p-Cl)在-108°C时,向先前报道的铀烷基络合物U(CH 2 C 6 H 5)4(2-Bn),U(CH 2- p -我PRC 6 ħ 4)4(2- p -我PR),U(CH 2 - p吨卜-C 6 H ^ 4)4(2- p -吨卜),U(CH 2 - ø - OMeC 6 H 4)4(2- o -OMe)和U(CH 2 – m -OMeC 6 H 4)4(2- m -OMe)。该家族的每个成员都容易与具有氧化还原活性的α-二亚胺配体Mes DAB Me(Mes DAB Me = [MesN═C(Me)C(Me)═NMes]; Mes = 2,4,6-三甲基苯基)反应,得到的产品从C-C还原消除,即(的Mes DAB我)U(CH 2 PH“)2和Ph'CH 2 CH 2 PH”(PH'= p -我PRC 6 ħ 4,p - t BuC 6 H 4,m -OMeC6 H 4,对-ClC 6 H 4)。当取代基的吸电子特性增加时,通过SQUID磁力测定法获得的室温磁化率值显示出与磁矩增加的相关性。动力学研究用于评估苄基取代基对还原消除速率的影响,表明反应速率随取代基吸电子性质的增加而增加。Eyring数据显示,熵值较大且为负,表明过渡态高度有序,这与先前报道的交叉实验得出的一致消除一致。
更新日期:2017-09-15
中文翻译:

均相铀烷基的协同CC消除反应的机理研究
进行了一项机械研究,以探讨从均相铀(IV)烷基中协同进行的CC还原消除。的对位-氯铀(IV)四苄衍生物,U(CH 2 - p -ClC 6 ħ 4)4(2- p -Cl),通过处理UCL合成4与4个当量的KCH 2 - p -Cl-PH (1-p-Cl)在-108°C时,向先前报道的铀烷基络合物U(CH 2 C 6 H 5)4(2-Bn),U(CH 2- p -我PRC 6 ħ 4)4(2- p -我PR),U(CH 2 - p吨卜-C 6 H ^ 4)4(2- p -吨卜),U(CH 2 - ø - OMeC 6 H 4)4(2- o -OMe)和U(CH 2 – m -OMeC 6 H 4)4(2- m -OMe)。该家族的每个成员都容易与具有氧化还原活性的α-二亚胺配体Mes DAB Me(Mes DAB Me = [MesN═C(Me)C(Me)═NMes]; Mes = 2,4,6-三甲基苯基)反应,得到的产品从C-C还原消除,即(的Mes DAB我)U(CH 2 PH“)2和Ph'CH 2 CH 2 PH”(PH'= p -我PRC 6 ħ 4,p - t BuC 6 H 4,m -OMeC6 H 4,对-ClC 6 H 4)。当取代基的吸电子特性增加时,通过SQUID磁力测定法获得的室温磁化率值显示出与磁矩增加的相关性。动力学研究用于评估苄基取代基对还原消除速率的影响,表明反应速率随取代基吸电子性质的增加而增加。Eyring数据显示,熵值较大且为负,表明过渡态高度有序,这与先前报道的交叉实验得出的一致消除一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号