当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoselective and Enantioselective Hydrogenation of 2,4‐Diaryl‐3H‐benzo[b]azepines Catalyzed by Dendritic Phosphinooxazoline Iridium Complexes
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-04-13 , DOI: 10.1002/ajoc.201700094 Tingting Miao 1, 2 , Baode Ma 1, 2 , Ziyuan Ding 1, 2 , Youran Liu 1, 2 , Yan-Mei He 1, 2 , Qing-Hua Fan 1, 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-04-13 , DOI: 10.1002/ajoc.201700094 Tingting Miao 1, 2 , Baode Ma 1, 2 , Ziyuan Ding 1, 2 , Youran Liu 1, 2 , Yan-Mei He 1, 2 , Qing-Hua Fan 1, 2
Affiliation
|
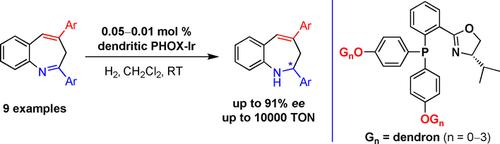
An asymmetric hydrogenation of 2,4‐diaryl‐3H‐benzo[b]azepines, catalyzed by dendritic phosphinooxazoline (PHOX) iridium complexes, has been developed. Only the imine group was reduced, while the C=C bond remained untouched, affording a range of chiral 2,3‐dihydro‐1H‐benzo[b]azepine derivatives with good to excellent enantioselectivity (up to 91 % ee). A slightly positive dendritic effect on catalytic reactivity (up to 10 000 TON) was observed.
中文翻译:

树枝状膦酰基恶唑啉铱配合物催化的2,4-二芳基-3H-苯并[b]氮杂环庚烷的化学选择和对映选择加氢反应
已经开发了由树枝状膦基恶唑啉(PHOX)铱络合物催化的2,4-二芳基-3 H-苯并[ b ]氮杂烃的不对称氢化。仅亚胺基被还原,而C = C键保持不变,从而提供了一系列具有良好至优异对映选择性(高达91%ee)的手性2,3-二氢-1H-苯并[ b ] a庚因衍生物。观察到对催化反应性有轻微的积极树突作用(高达10000吨)。
更新日期:2017-04-13
中文翻译:

树枝状膦酰基恶唑啉铱配合物催化的2,4-二芳基-3H-苯并[b]氮杂环庚烷的化学选择和对映选择加氢反应
已经开发了由树枝状膦基恶唑啉(PHOX)铱络合物催化的2,4-二芳基-3 H-苯并[ b ]氮杂烃的不对称氢化。仅亚胺基被还原,而C = C键保持不变,从而提供了一系列具有良好至优异对映选择性(高达91%ee)的手性2,3-二氢-1H-苯并[ b ] a庚因衍生物。观察到对催化反应性有轻微的积极树突作用(高达10000吨)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号