Tetrahedron Letters ( IF 1.8 ) Pub Date : 2017-09-14 , DOI: 10.1016/j.tetlet.2017.09.026 Innus Mohammad , JiYoung Mun , Amber Onorato , Martha D. Morton , Abdullah I. Saleh , Michael B. Smith
|
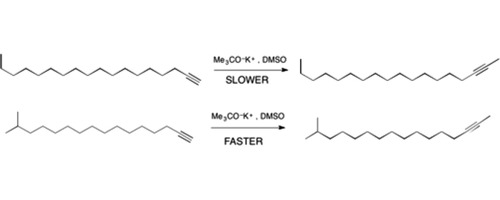
When compared to a long-straight chain terminal alkyne, a long chain terminal alkyne with a distal isopropyl unit (isobranched) isomerizes about two times faster when treated with strong base under identical conditions, and appears to follow pseudo first order kinetics. In both cases, equilibration to a 95–97:5–3 mixture of terminal:internal alkyne accompanies isomerization. The difference in rate may be due to an unusual folding of both long-chain alkynes, bringing the distal substituent close to the carbon-carbon-triple bond moiety. The distal isopropyl moiety may provide unanticipated steric hindrance that disrupts such folding, making the propargylic proton more available for reaction with base.
中文翻译:

远端取代对长链末端炔烃碱基诱导的异构化的影响
与长直链末端炔烃相比,带有远端异丙基单元(异支链)的长链末端炔烃在相同条件下用强碱处理时异构化速度快约两倍,并且似乎遵循伪一级动力学。在两种情况下,异构化都会使末端炔烃:内部炔烃平衡至95–97:5–3的混合物达到平衡。速率差异可能是由于两个长链炔烃异常折叠所致,使远端取代基接近碳-碳-三键部分。远端异丙基部分可能会提供无法预料的空间位阻,从而破坏此类折叠,从而使炔丙基质子更易于与碱反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号