Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-09-14 , DOI: 10.1016/j.tetlet.2017.09.033 Sreedhar Reddy Tummalapalli , Rohit Bhat , Craig Waitt , Henk Eshuis , David P. Rotella
|
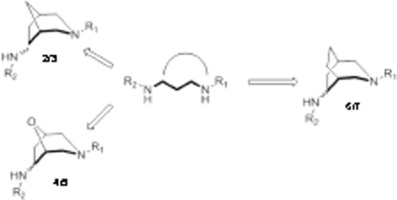
Conformational restriction is a useful approach for ligand design in organic and medicinal chemistry. This manuscript reports the facile synthesis and in silico conformational analysis of two new diastereomeric [3.2.2]-3-azabicyclic, two new [3.2.1]-3-aza-8-oxy-bicyclic and one new [3.2.1]-3-azabicyclic diamine scaffolds. A conformational analysis of these structures along with calculation of carbon–carbon/carbon–nitrogen bond angles was carried out and compared to those in the flexible 1,3-diaminopropane template upon which they were based. It is of particular importance that these scaffolds have bond lengths and angles that can overlap with low energy conformers of the flexible diamine. Such information is useful for ligand design in organic chemistry and for development of structure activity relationships and in silico screening in medicinal chemistry.
中文翻译:

构象受限的[3.2.2]-和[3.2.1] -3-氮杂双环二胺的合成和计算分析
构象限制是有机和药物化学中配体设计的有用方法。该手稿报告了简便的合成和计算机模拟两个新的非对映异构[3.2.2] -3-氮杂双环,两个新的[3.2.1] -3-氮杂-8-氧-双环和一个新的[3.2.1] -3-氮杂双环二胺骨架的构象分析。对这些结构进行了构象分析,并计算了碳-碳/碳-氮键角,并与它们所基于的灵活的1,3-二氨基丙烷模板中的结构进行了比较。尤其重要的是这些支架具有可与柔性二胺的低能构象体重叠的键长和键角。这些信息对于有机化学中的配体设计以及结构活性关系的发展以及药物化学中的计算机筛选有用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号