Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Slp1-Emp65: A Guardian Factor that Protects Folding Polypeptides from Promiscuous Degradation.
Cell ( IF 45.5 ) Pub Date : 2017-Oct-05 , DOI: 10.1016/j.cell.2017.08.036 Shan Zhang , Chengchao Xu , Katherine E. Larrimore , Davis T.W. Ng
Cell ( IF 45.5 ) Pub Date : 2017-Oct-05 , DOI: 10.1016/j.cell.2017.08.036 Shan Zhang , Chengchao Xu , Katherine E. Larrimore , Davis T.W. Ng
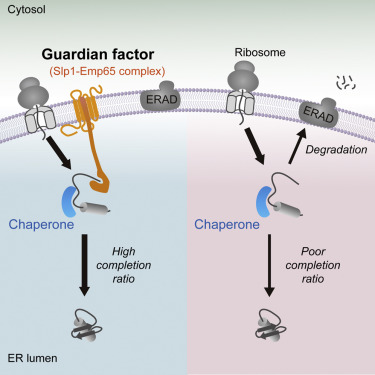
|
Newly synthesized proteins engage molecular chaperones that assist folding. Their progress is monitored by quality control systems that target folding errors for degradation. Paradoxically, chaperones that promote folding also direct unfolded polypeptides for degradation. Hence, a mechanism was previously hypothesized that prevents the degradation of actively folding polypeptides. In this study, we show that a conserved endoplasmic reticulum (ER) membrane protein complex, consisting of Slp1 and Emp65 proteins, performs this function in the ER lumen. The complex binds unfolded proteins and protects them from degradation during folding. In its absence, approximately 20%-30% of newly synthesized proteins that could otherwise fold are degraded. Although the Slp1-Emp65 complex hosts a broad range of clients, it is specific for soluble proteins. Taken together, these studies demonstrate the vulnerability of newly translated, actively folding polypeptides and the discovery of a new proteostasis functional class we term "guardian" that protects them from degradation.
中文翻译:

Slp1-Emp65:保护折叠多肽免于混杂降解的保护因子。
新合成的蛋白质与辅助折叠的分子伴侣结合。其进度由质量控制系统监控,该系统以折叠错误为目标。矛盾的是,促进折叠的分子伴侣也指导未折叠的多肽降解。因此,先前假设了一种防止主动折叠多肽降解的机制。在这项研究中,我们表明保守的内质网(ER)膜蛋白复合物,由Slp1和Emp65蛋白质组成,在ER管腔中执行此功能。该复合物结合未折叠的蛋白质并保护它们在折叠过程中不被降解。在没有它的情况下,原本可以折叠的新合成蛋白质中约有20%-30%被降解。尽管Slp1-Emp65复合物拥有广泛的客户,但它对可溶性蛋白质具有特异性。
更新日期:2017-09-15
中文翻译:

Slp1-Emp65:保护折叠多肽免于混杂降解的保护因子。
新合成的蛋白质与辅助折叠的分子伴侣结合。其进度由质量控制系统监控,该系统以折叠错误为目标。矛盾的是,促进折叠的分子伴侣也指导未折叠的多肽降解。因此,先前假设了一种防止主动折叠多肽降解的机制。在这项研究中,我们表明保守的内质网(ER)膜蛋白复合物,由Slp1和Emp65蛋白质组成,在ER管腔中执行此功能。该复合物结合未折叠的蛋白质并保护它们在折叠过程中不被降解。在没有它的情况下,原本可以折叠的新合成蛋白质中约有20%-30%被降解。尽管Slp1-Emp65复合物拥有广泛的客户,但它对可溶性蛋白质具有特异性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号