Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
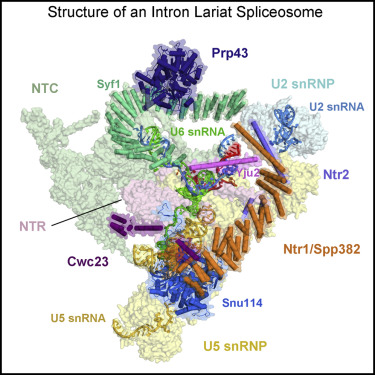
Structure of an Intron Lariat Spliceosome from Saccharomyces cerevisiae.
Cell ( IF 45.5 ) Pub Date : 2017-Sep-21 , DOI: 10.1016/j.cell.2017.08.029 Ruixue Wan , Chuangye Yan , Rui Bai , Jianlin Lei , Yigong Shi
Cell ( IF 45.5 ) Pub Date : 2017-Sep-21 , DOI: 10.1016/j.cell.2017.08.029 Ruixue Wan , Chuangye Yan , Rui Bai , Jianlin Lei , Yigong Shi
|
The disassembly of the intron lariat spliceosome (ILS) marks the end of a splicing cycle. Here we report a cryoelectron microscopy structure of the ILS complex from Saccharomyces cerevisiae at an average resolution of 3.5 Å. The intron lariat remains bound in the spliceosome whereas the ligated exon is already dissociated. The step II splicing factors Prp17 and Prp18, along with Cwc21 and Cwc22 that stabilize the 5' exon binding to loop I of U5 small nuclear RNA (snRNA), have been released from the active site assembly. The DEAH family ATPase/helicase Prp43 binds Syf1 at the periphery of the spliceosome, with its RNA-binding site close to the 3' end of U6 snRNA. The C-terminal domain of Ntr1/Spp382 associates with the GTPase Snu114, and Ntr2 is anchored to Prp8 while interacting with the superhelical domain of Ntr1. These structural features suggest a plausible mechanism for the disassembly of the ILS complex.
中文翻译:

来自酿酒酵母的内含子套索剪接体的结构。
内含子套索剪接体(ILS)的拆卸标志着剪接循环的结束。在这里,我们报道了来自酿酒酵母的ILS复合物的低温电子显微镜结构,平均分辨率为3.5。内含子套索保持结合在剪接体中,而连接的外显子已经解离。步骤II的剪接因子Prp17和Prp18,以及稳定5'外显子与U5小核RNA(snRNA)环I的5'外显子结合的Cwc21和Cwc22,已经从活性位点组件中释放出来。DEAH家族ATPase /解旋酶Prp43在剪接体外围结合Syf1,其RNA结合位点靠近U6 snRNA的3'端。Ntr1 / Spp382的C末端结构域与GTPase Snu114关联,并且Ntr2在与Ntr1的超螺旋结构域相互作用的同时锚定到Prp8。
更新日期:2017-09-15
中文翻译:

来自酿酒酵母的内含子套索剪接体的结构。
内含子套索剪接体(ILS)的拆卸标志着剪接循环的结束。在这里,我们报道了来自酿酒酵母的ILS复合物的低温电子显微镜结构,平均分辨率为3.5。内含子套索保持结合在剪接体中,而连接的外显子已经解离。步骤II的剪接因子Prp17和Prp18,以及稳定5'外显子与U5小核RNA(snRNA)环I的5'外显子结合的Cwc21和Cwc22,已经从活性位点组件中释放出来。DEAH家族ATPase /解旋酶Prp43在剪接体外围结合Syf1,其RNA结合位点靠近U6 snRNA的3'端。Ntr1 / Spp382的C末端结构域与GTPase Snu114关联,并且Ntr2在与Ntr1的超螺旋结构域相互作用的同时锚定到Prp8。











































 京公网安备 11010802027423号
京公网安备 11010802027423号