PLOS Neglected Tropical Diseases ( IF 3.4 ) Pub Date : 2017-09-14 , DOI: 10.1371/journal.pntd.0005794 Jonathan D. Alpern , Rogelio Lopez-Velez , William M. Stauffer
|
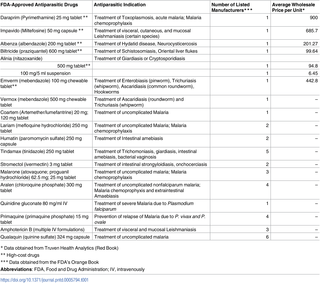
Drugs for neglected tropical diseases (NTD) are being excessively priced in the United States. Benznidazole, the first-line drug for Chagas disease, may become approved by the Food and Drug Administration (FDA) and manufactured by a private company in the US, thus placing it at risk of similar pricing. Chagas disease is an NTD caused by Trypanosoma cruzi; it is endemic to Latin America, infecting 8 million individuals. Human migration has changed the epidemiology causing nonendemic countries to face increased challenges in diagnosing and managing patients with Chagas disease. Only 2 drugs exist with proven efficacy: benznidazole and nifurtimox. Benznidazole has historically faced supply problems and drug shortages, limiting accessibility. In the US, it is currently only available under an investigational new drug (IND) protocol from the CDC and is provided free of charge to patients. However, 2 companies have stated that they intend to submit a New Drug Application (NDA) for FDA approval. Based on recent history of companies acquiring licensing rights for NTD drugs in the US with limited availability, it is likely that benznidazole will become excessively priced by the manufacturer—paradoxically making it less accessible. However, if the companies can be taken at their word, there may be reason for optimism.
中文翻译:

在美国恰加斯病中使用苯并硝唑治疗是否持谨慎乐观的态度?
在美国,用于被忽视的热带病(NTD)的药物定价过高。苯甲唑是查加斯病的一线药物,可能会获得美国食品药品监督管理局(FDA)的批准,并由美国一家私人公司生产,因此有可能面临类似价格的风险。恰加斯病是由克鲁氏锥虫引起的NTD; 它是拉丁美洲的特有疾病,感染了800万人。人口迁移改变了流行病学,导致非流行国家在诊断和管理恰加斯病患者方面面临越来越多的挑战。仅存在2种已证明具有疗效的药物:苯并硝唑和硝呋替莫。苯甲唑在历史上一直面临供应问题和药品短缺,限制了药物的可及性。在美国,目前仅可根据CDC的研究性新药(IND)协议使用它,并且免费向患者提供。但是,有2家公司表示他们打算提交新药申请(NDA)以获得FDA批准。根据最近在美国获得NTD药品许可权且数量有限的公司的历史,苯并硝唑很可能会被制造商定价过高,反而使其难以获得。但是,如果能如愿以偿地对待公司,则可能有理由感到乐观。











































 京公网安备 11010802027423号
京公网安备 11010802027423号