PLoS Pathogens ( IF 5.5 ) Pub Date : 2017-09-14 , DOI: 10.1371/journal.ppat.1006623 Bradley R. Groveman , Gregory J. Raymond , Katrina J. Campbell , Brent Race , Lynne D. Raymond , Andrew G. Hughson , Christina D. Orrú , Allison Kraus , Katie Phillips , Byron Caughey
|
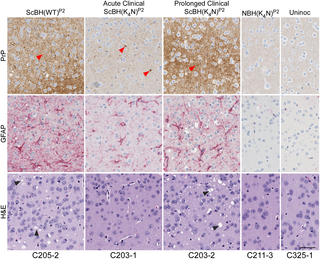
Mammalian prion structures and replication mechanisms are poorly understood. Most synthetic recombinant prion protein (rPrP) amyloids prepared without cofactors are non-infectious or much less infectious than bona fide tissue-derived PrPSc. This effect has been associated with differences in folding of the aggregates, manifested in part by reduced solvent exclusion and protease-resistance in rPrP amyloids, especially within residues ~90–160. Substitution of 4 lysines within residues 101–110 of rPrP (central lysine cluster) with alanines (K4A) or asparagines (K4N) allows formation of aggregates with extended proteinase K (PK) resistant cores reminiscent of PrPSc, particularly when seeded with PrPSc. Here we have compared the infectivity of rPrP aggregates made with K4N, K4A or wild-type (WT) rPrP, after seeding with scrapie brain homogenate (ScBH) or normal brain homogenate (NBH). None of these preparations caused clinical disease on first passage into rodents. However, the ScBH-seeded fibrils (only) led to a subclinical pathogenesis as indicated by increases in prion seeding activity, neuropathology, and abnormal PrP in the brain. Seeding activities usually accumulated to much higher levels in animals inoculated with ScBH-seeded fibrils made with the K4N, rather than WT, rPrP molecules. Brain homogenates from subclinical animals induced clinical disease on second passage into “hamsterized” Tg7 mice, with shorter incubation times in animals inoculated with ScBH-seeded K4N rPrP fibrils. On second passage from animals inoculated ScBH-seeded WT fibrils, we detected an additional PK resistant PrP fragment that was similar to that of bona fide PrPSc. Together these data indicate that both the central lysine cluster and scrapie seeding of rPrP aggregates influence the induction of PrP misfolding, neuropathology and clinical manifestations upon passage in vivo. We confirm that some rPrP aggregates can initiate further aggregation without typical pathogenesis in vivo. We also provide evidence that there is little, if any, biohazard associated with routine RT-QuIC assays.
中文翻译:

中央赖氨酸簇和瘙痒病模板在合成病毒蛋白聚集体可传递性中的作用
哺乳动物病毒的结构和复制机制了解甚少。制备的没有辅因子的大多数合成重组病毒蛋白(rPrP)淀粉样蛋白均无感染性,或与真正组织衍生的PrP Sc相比无感染性。这种作用与聚集体折叠的差异有关,部分表现为rPrP淀粉样蛋白的溶剂排斥和蛋白酶抗性降低,尤其是在〜90-160的残基中。用丙氨酸(K 4 A)或天冬酰胺(K 4 N)取代rPrP(中央赖氨酸簇)的残基101–110中的4个赖氨酸,可形成具有延伸的蛋白酶K(PK)抗性核心的聚集体,使人联想到PrP Sc,尤其是当用PrP播种SC。在这里,我们比较了用痒痒性脑匀浆(ScBH)或正常脑匀浆(NBH)播种后,用K 4 N,K 4 A或野生型(WT)rPrP制成的rPrP聚集体的感染性。这些制剂在首次进入啮齿动物中均未引起临床疾病。然而,ScBH播种的原纤维(仅)导致亚临床发病机制,如病毒播种活性,神经病理学和脑内PrP异常增加所表明。通常,接种用K 4制成的ScBH接种原纤维的动物中的播种活动积累的水平通常更高。N,而不是WT rPrP分子。来自亚临床动物的脑匀浆在第二次传给“仓鼠” Tg7小鼠后诱发了临床疾病,在接种了ScBH接种的K 4 N rPrP原纤维的动物中,孵化时间更短。从接种了ScBH种子的WT原纤维的动物第二次传代后,我们检测到了另一个与真正的PrP Sc相似的抗PK的PrP片段。这些数据加在一起表明,rPrP聚集体的中央赖氨酸簇和羊皮草接种均会影响在体内通过时对PrP错折叠,神经病理学和临床表现的诱导。我们证实某些rPrP聚集体可以启动进一步的聚集,而无需体内典型的发病机制。我们还提供了证据,表明与常规RT-QuIC分析相关的生物危害很小(如果有的话)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号