当前位置:
X-MOL 学术
›
Anal. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of a trap-free two-dimensional liquid chromatography combined with ion trap/time-of-flight mass spectrometry for separation and characterization of impurities and isomers in cefpiramide
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.aca.2017.08.028 Jian Wang , Yu Xu , Chunmei Wen , Zhijian Wang
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.aca.2017.08.028 Jian Wang , Yu Xu , Chunmei Wen , Zhijian Wang
|
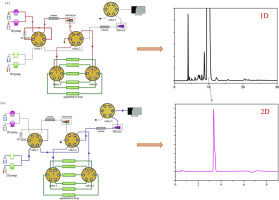
High-resolution mass spectrometry had been routinely used for structure identification of impurity. However, all LC-MS methods were based on a volatile mobile phase, and a non-volatile system is used in the official analytical method of United States Pharmacopoeia for cefpiramide which limited the use of mass spectrometry for structure characterization of the impurities. Here we presented the utilization of a trap-free two-dimensional liquid chromatography coupled to high resolution ion trap/time-of-flight mass spectrometry (2D LC-IT-TOF MS) with positive and negative modes of electrospray ionization for characterization of eight impurities in cefpiramide. Trap-free two-dimensional liquid chromatography and online desalting technique made it possible to characterize the impurity in cefpiramide in the condition of official standard, and the TIC chromatogram of LC-MS was in conformity with the LC chromatogram of the official analytical method in the peak sequence of impurities, which could further improve the method of official monographs in pharmacopoeias. Each peak separated by the non-volatile mobile phase was trapped by a 20 μL quantitative loop then transferred into a system with a volatile mobile phase connected to a MS detector. In the first dimension, the column was Kromasil C8 analytical column (250 mm × 4.6 mm, 5 μm) with a non-volatile salt mobile phase at the flow rate of 0.8 mL min-1. In the second dimension, the column was Shimadzu Shim-pack GISS C18 (50 mm × 2.1 mm, 1.9 μm) with a volatile salt mobile phase at the flow rate of 0.3 mL min-1. Through the multiple heart-cutting 2D-LC approach and online desalting technique, the problem of incompatibility between non-volatile salt mobile phase and mass spectrometry was solved completely. The fragmentation behavior of cefpiramide and its eight impurities were studied. The structures of eight impurities in cefpiramide drug substance were deduced based on the HPLC-MSn data, in which seven impurities were novel impurities. The forming mechanisms of degradation products in cefpiramide were also studied.
中文翻译:

无阱二维液相色谱结合离子阱/飞行时间质谱在头孢匹胺中杂质和异构体分离表征中的应用
高分辨质谱已常规用于杂质的结构鉴定。然而,所有 LC-MS 方法均基于挥发性流动相,并且美国药典官方头孢匹胺分析方法使用非挥发性系统,这限制了质谱法用于杂质结构表征的使用。在这里,我们介绍了使用无阱二维液相色谱与高分辨率离子阱/飞行时间质谱 (2D LC-IT-TOF MS) 结合的正负电喷雾电离模式,用于表征八个头孢匹胺中的杂质。无陷阱二维液相色谱和在线脱盐技术使得在官方标准条件下对头孢匹胺中的杂质进行表征成为可能,LC-MS的TIC色谱图在杂质峰序列上与官方分析方法的LC色谱图一致,可进一步完善药典官方专论的方法。由非挥发性流动相分离的每个峰都被 20 μL 定量环捕获,然后转移到一个系统中,挥发性流动相连接到 MS 检测器。在第一维中,色谱柱为 Kromasil C8 分析柱(250 mm × 4.6 mm,5 μm),流动相为非挥发性盐,流速为 0.8 mL min-1。在第二维中,色谱柱是 Shimadzu Shim-pack GISS C18(50 mm × 2.1 mm,1.9 μm),流动相为 0.3 mL min-1 的挥发性盐。通过多中心切割二维液相色谱方法和在线脱盐技术,彻底解决了非挥发性盐类流动相与质谱不兼容的问题。研究了头孢匹胺及其八种杂质的碎裂行为。根据HPLC-MSn数据推导出头孢匹胺原料药中8种杂质的结构,其中7种杂质为新杂质。还研究了头孢匹胺中降解产物的形成机制。
更新日期:2017-11-01
中文翻译:

无阱二维液相色谱结合离子阱/飞行时间质谱在头孢匹胺中杂质和异构体分离表征中的应用
高分辨质谱已常规用于杂质的结构鉴定。然而,所有 LC-MS 方法均基于挥发性流动相,并且美国药典官方头孢匹胺分析方法使用非挥发性系统,这限制了质谱法用于杂质结构表征的使用。在这里,我们介绍了使用无阱二维液相色谱与高分辨率离子阱/飞行时间质谱 (2D LC-IT-TOF MS) 结合的正负电喷雾电离模式,用于表征八个头孢匹胺中的杂质。无陷阱二维液相色谱和在线脱盐技术使得在官方标准条件下对头孢匹胺中的杂质进行表征成为可能,LC-MS的TIC色谱图在杂质峰序列上与官方分析方法的LC色谱图一致,可进一步完善药典官方专论的方法。由非挥发性流动相分离的每个峰都被 20 μL 定量环捕获,然后转移到一个系统中,挥发性流动相连接到 MS 检测器。在第一维中,色谱柱为 Kromasil C8 分析柱(250 mm × 4.6 mm,5 μm),流动相为非挥发性盐,流速为 0.8 mL min-1。在第二维中,色谱柱是 Shimadzu Shim-pack GISS C18(50 mm × 2.1 mm,1.9 μm),流动相为 0.3 mL min-1 的挥发性盐。通过多中心切割二维液相色谱方法和在线脱盐技术,彻底解决了非挥发性盐类流动相与质谱不兼容的问题。研究了头孢匹胺及其八种杂质的碎裂行为。根据HPLC-MSn数据推导出头孢匹胺原料药中8种杂质的结构,其中7种杂质为新杂质。还研究了头孢匹胺中降解产物的形成机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号