当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tandem Synthesis of Pyrrolo[2,3-b]quinolones via Cadogen-Type Reaction
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acs.orglett.7b02558 Zhichen Lin 1 , Zhongyan Hu 1, 2 , Xin Zhang 2 , Jinhuan Dong 2 , Jian-Biao Liu 2 , De-Zhan Chen 2 , Xianxiu Xu 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acs.orglett.7b02558 Zhichen Lin 1 , Zhongyan Hu 1, 2 , Xin Zhang 2 , Jinhuan Dong 2 , Jian-Biao Liu 2 , De-Zhan Chen 2 , Xianxiu Xu 1, 2
Affiliation
|
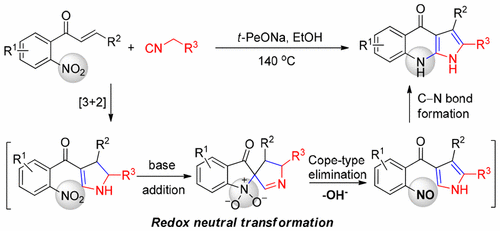
A tandem [3 + 2] cycloaddition/reductive cyclization of nitrochalcones with activated methylene isocyanides for the efficient synthesis of pyrrolo[2,3-b]quinolones is reported. In this reaction, the in situ generated dihydropyrroline acts as the internal reductant to convert the nitro into an electrophilic nitroso group, which undergoes subsequent C–N bond formation. Transition-metal-free, simple experimental procedure and ready accessibility of starting materials characterize the present transformation.
中文翻译:

通过Cadogen型反应串联合成吡咯并[2,3- b ]喹诺酮
报道了用活化的亚甲基异氰酸酯串联硝唑烷的[3 + 2]环加成/还原环化反应,可有效合成吡咯并[2,3- b ]喹诺酮。在该反应中,原位生成的二氢吡咯啉充当内部还原剂,将硝基转化为亲电的亚硝基,随后发生C–N键的形成。不含过渡金属,简单的实验程序以及易于使用的起始材料构成了目前的转化方式。
更新日期:2017-09-14
中文翻译:

通过Cadogen型反应串联合成吡咯并[2,3- b ]喹诺酮
报道了用活化的亚甲基异氰酸酯串联硝唑烷的[3 + 2]环加成/还原环化反应,可有效合成吡咯并[2,3- b ]喹诺酮。在该反应中,原位生成的二氢吡咯啉充当内部还原剂,将硝基转化为亲电的亚硝基,随后发生C–N键的形成。不含过渡金属,简单的实验程序以及易于使用的起始材料构成了目前的转化方式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号