当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of the Large-Scale Synthesis of Tetrahydropyran Glycine, a Precursor to the HCV NS5A Inhibitor BMS-986097
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acs.joc.7b01852 Arvind Mathur 1 , Bei Wang 1 , Daniel Smith 1 , Jianqing Li 1 , Joseph Pawluczyk 1 , Jung-Hui Sun 1 , Michael Kwok Wong 1 , Subramaniam Krishnananthan 1 , Dauh-Rurng Wu 1 , Dawn Sun 1 , Peng Li 1 , Shiuhang Yip 1 , Bang-Chi Chen 1 , Phil S. Baran 2 , Qi Chen 3 , Omar D. Lopez 3 , Zhong Yong 3 , John A. Bender 3 , Van N. Nguyen 3 , Jeffrey L. Romine 3 , Denis R. St. Laurent 3 , Gan Wang 3 , John F. Kadow 3 , Nicholas A. Meanwell 3 , Makonen Belema 3 , Rulin Zhao 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acs.joc.7b01852 Arvind Mathur 1 , Bei Wang 1 , Daniel Smith 1 , Jianqing Li 1 , Joseph Pawluczyk 1 , Jung-Hui Sun 1 , Michael Kwok Wong 1 , Subramaniam Krishnananthan 1 , Dauh-Rurng Wu 1 , Dawn Sun 1 , Peng Li 1 , Shiuhang Yip 1 , Bang-Chi Chen 1 , Phil S. Baran 2 , Qi Chen 3 , Omar D. Lopez 3 , Zhong Yong 3 , John A. Bender 3 , Van N. Nguyen 3 , Jeffrey L. Romine 3 , Denis R. St. Laurent 3 , Gan Wang 3 , John F. Kadow 3 , Nicholas A. Meanwell 3 , Makonen Belema 3 , Rulin Zhao 1
Affiliation
|
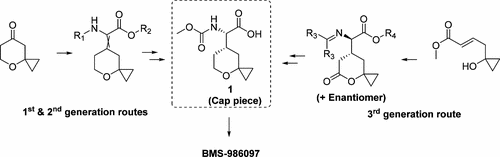
An efficient large-scale synthesis of acid 1, a penultimate precursor to the HCV NS5A inhibitor BMS-986097, along with the final API step are described. Three routes were devised for the synthesis of 1 at the various stages of the program. The third generation route, the one that proved scalable and is the main subject of this paper, features a one-step Michael addition of t-butyl 2-((diphenylmethylene)amino)acetate (24) to (E)-benzyl 4-(1-hydroxycyclopropyl)but-2-enoate (28) followed by cyclization and chiral separation to form 27c, the core skeleton of cap piece 1. The epimerization and chiral resolution of 27c followed by further synthetic manipulations involving the carbamate formation, lactone reduction and cyclization, afforded cyclopropyl pyran 1. A detailed study of diphenylmethane deprotection via acid hydrolysis as well as a key lactone to tetrahydropyran conversion, in order to avoid a side reaction that afforded an alternative cyclization product, are discussed. This synthesis was applied to the preparation of more than 100 g of the final API BMS-986097 for toxicology studies.
中文翻译:

HCV NS5A抑制剂BMS-986097的前体四氢吡喃甘氨酸的大规模合成方法的开发
描述了有效的大规模合成酸1的方法,它是HCV NS5A抑制剂BMS-986097的倒数第二个前体,以及最后的API步骤。在程序的各个阶段,设计了三种合成1的途径。第三代途径,证明是可扩展的,是本文的主要主题,其特征在于,将2-(((二苯基亚甲基)氨基)乙酸叔丁酯(24)与(E)-4-苄基进行一步一步迈克尔加成反应。(1-羟基环丙基)丁-2-烯酸酯(28),然后环化和手性分离,形成27c(帽盖1的核心骨架)。27c的差向异构和手性拆分,再进行涉及氨基甲酸酯形成,内酯还原和环化的进一步合成操作,得到环丙基吡喃1。讨论了通过酸水解对二苯甲烷脱保护以及关键的内酯向四氢吡喃转化的详细研究,以避免产生可替代的环化产物的副反应。该合成用于制备100克以上的用于毒理学研究的最终API BMS-986097。
更新日期:2017-09-15
中文翻译:

HCV NS5A抑制剂BMS-986097的前体四氢吡喃甘氨酸的大规模合成方法的开发
描述了有效的大规模合成酸1的方法,它是HCV NS5A抑制剂BMS-986097的倒数第二个前体,以及最后的API步骤。在程序的各个阶段,设计了三种合成1的途径。第三代途径,证明是可扩展的,是本文的主要主题,其特征在于,将2-(((二苯基亚甲基)氨基)乙酸叔丁酯(24)与(E)-4-苄基进行一步一步迈克尔加成反应。(1-羟基环丙基)丁-2-烯酸酯(28),然后环化和手性分离,形成27c(帽盖1的核心骨架)。27c的差向异构和手性拆分,再进行涉及氨基甲酸酯形成,内酯还原和环化的进一步合成操作,得到环丙基吡喃1。讨论了通过酸水解对二苯甲烷脱保护以及关键的内酯向四氢吡喃转化的详细研究,以避免产生可替代的环化产物的副反应。该合成用于制备100克以上的用于毒理学研究的最终API BMS-986097。










































 京公网安备 11010802027423号
京公网安备 11010802027423号