Sensors and Actuators B: Chemical ( IF 8.0 ) Pub Date : 2017-09-14 , DOI: 10.1016/j.snb.2017.09.087 Fanping Shi , Lei Wang , Yan Li , Yu Zhang , Xingguang Su
|
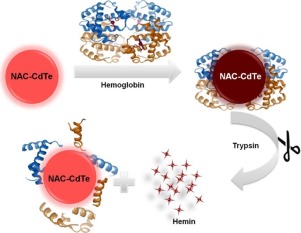
In this work, we designed a simple, convenient and high sensitive “turn-on” fluorescence platform for trypsin assay based on N-acetyl-l-cysteine capped CdTe Quantum Dots (NAC-CdTe QDs). A series of NAC-CdTe QDs with different sizes were synthesized in aqueous via refluxing routes. The fluorescence of NAC-CdTe QDs could be effectively quenched by hemoglobin (Hb) via the electron transfer interactions from NAC-CdTe QDs to hemoglobin due to the electrostatic attraction of QDs/Hb complex. In the presence of trypsin, hemoglobin would be hydrolyzed to small peptides, releasing inactive hemin molecules.Hemin has slight effect on the fluorescent intensity of NAC-CdTe QDs, so the combination of hemoglobin and NAC-CdTe QDs would be dissociated, leading to the fluorescence recovery of NAC-CdTe QDs. Therefore, we could monitor the trypsin activity by utilizing the different fluoresence responses of NAC-CdTe QDs to hemoglobin and hemin. The recovered fluorescence intensity of NAC-CdTe QDs was proportional to the logarithm of trypsin concentration in the range of 0.05–10 mU mL−1 and the detection limit for trypsin was 0.036 mU mL−1. A model for trypsin inhibition was further established to verify the feasibility of the system. The IC50 value is 3.06 μg mL−1 for the inhibitor from soybean. Thus, a label free real time fluorometric assay for trypsin and inhibitor screening has been developed. The established method showed a high selectivity and sensitivity for trypsin over other biological relevant enzyme, and it was applied to the determination of trypsin in human urine sample with satisfactory results.
中文翻译:

一个简单的“开启”检测平台,用于基于N-乙酰基-1-半胱氨酸加帽的CdTe量子点的胰蛋白酶活性和抑制剂筛选
在这项工作中,我们设计了一种简单,方便且高灵敏度的“开启”荧光平台,用于基于N-乙酰基-l的胰蛋白酶测定-半胱氨酸封端的CdTe量子点(NAC-CdTe QD)。通过回流途径在水溶液中合成了一系列大小不同的NAC-CdTe QD。由于QDs / Hb络合物的静电吸引作用,通过NAC-CdTe QDs与血红蛋白的电子转移相互作用,血红蛋白(Hb)可以有效地抑制NAC-CdTe QDs的荧光。在存在胰蛋白酶的情况下,血红蛋白会水解成小肽,释放出失活的血红素分子。血红素对NAC-CdTe QDs的荧光强度影响很小,因此血红蛋白和NAC-CdTe QDs的结合会解离,从而导致NAC-CdTe QD的荧光恢复。因此,我们可以利用NAC-CdTe QD对血红蛋白和血红素的不同荧光反应来监测胰蛋白酶的活性。-1,胰蛋白酶的检出限为0.036 mU mL -1。进一步建立了胰蛋白酶抑制模型,以验证该系统的可行性。来自大豆的抑制剂的IC 50值为3.06μgmL -1。因此,已经开发了用于胰蛋白酶和抑制剂筛选的无标记实时荧光测定法。建立的方法对胰蛋白酶对其他生物相关酶具有很高的选择性和敏感性,可用于人尿样中胰蛋白酶的测定,结果令人满意。










































 京公网安备 11010802027423号
京公网安备 11010802027423号