当前位置:
X-MOL 学术
›
Chem. Phys. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Reactivity of C(1D) with Oxygen Bearing Molecules NO and O2 at Low Temperature
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2017-09-14 , DOI: 10.1016/j.cplett.2017.09.028 Dianailys Nuñez-Reyes , Kevin M. Hickson
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2017-09-14 , DOI: 10.1016/j.cplett.2017.09.028 Dianailys Nuñez-Reyes , Kevin M. Hickson
|
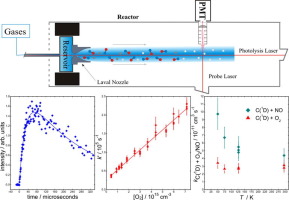
The gas-phase reactions, C(1D) + NO and C(1D) + O2 have been studied over the 50-296 K range using a Laval nozzle reactor. C(1D) atoms were produced by CBr4 photolysis. A tracer technique was employed to follow the kinetics by adding H2 or CH4 to the flow. H-atoms produced by the C(1D) + H2/CH4 reactions were detected by laser-induced fluorescence. Rate constants for the C(1D) + O2 reaction show little variation with temperature, whereas rate constants for the C(1D) + NO reaction increase rapidly below 100 K. Numerical simulations were performed to evaluate the influence of secondary reactions.
中文翻译:

C(的反应1 d)与氧分子轴承NO和O 2在低温
使用拉瓦尔喷嘴反应器在50-296 K范围内研究了气相反应C(1 D)+ NO和C(1 D)+ O 2。通过CBr 4光解产生C(1 D)原子。通过向流中添加H 2或CH 4,采用示踪技术跟踪动力学。由C(1 D)+ H 2 / CH 4反应产生的H原子通过激光诱导的荧光进行检测。C(1 D)+ O 2反应的速率常数随温度变化不大,而C(1D)+ NO反应在100 K以下迅速增加。进行了数值模拟,以评估副反应的影响。
更新日期:2017-09-15
中文翻译:

C(的反应1 d)与氧分子轴承NO和O 2在低温
使用拉瓦尔喷嘴反应器在50-296 K范围内研究了气相反应C(1 D)+ NO和C(1 D)+ O 2。通过CBr 4光解产生C(1 D)原子。通过向流中添加H 2或CH 4,采用示踪技术跟踪动力学。由C(1 D)+ H 2 / CH 4反应产生的H原子通过激光诱导的荧光进行检测。C(1 D)+ O 2反应的速率常数随温度变化不大,而C(1D)+ NO反应在100 K以下迅速增加。进行了数值模拟,以评估副反应的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号