Journal of Controlled Release ( IF 10.5 ) Pub Date : 2017-09-14 , DOI: 10.1016/j.jconrel.2017.09.019 Helena Costa Verdera , Jerney J. Gitz-Francois , Raymond M. Schiffelers , Pieter Vader
|
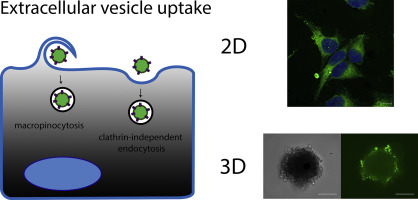
Recent evidence has established that extracellular vesicles (EVs), including exosomes and microvesicles, form an endogenous transport system through which biomolecules, including proteins and RNA, are exchanged between cells. This endows EVs with immense potential for drug delivery and regenerative medicine applications. Understanding the biology underlying EV-based intercellular transfer of cargo is of great importance for the development of EV-based therapeutics. Here, we sought to characterize the cellular mechanisms involved in EV uptake.
Internalization of fluorescently-labeled EVs was evaluated in HeLa cells, in 2D (monolayer) cell culture as well as 3D spheroids. Uptake was assessed using flow cytometry and confocal microscopy, using chemical as well as RNA interference-based inhibition of key proteins involved in individual endocytic pathways. Experiments with chemical inhibitors revealed that EV uptake depends on cholesterol and tyrosine kinase activity, which are implicated in clathrin-independent endocytosis, and on Na+/H+ exchange and phosphoinositide 3-kinase activity, which are important for macropinocytosis. Furthermore, EV internalization was inhibited by siRNA-mediated knockdown of caveolin-1, flotillin-1, RhoA, Rac1 and PAK1, but not clathrin heavy chain. Together, these results suggest that EVs enter cells predominantly via clathrin-independent endocytosis and macropinocytosis. Identification of EV components that promote their uptake via pathways that lead to functional cargo transfer might allow development of more efficient therapeutics through EV-inspired engineering.
中文翻译:

细胞外囊泡的细胞摄取是由网格蛋白非依赖性内吞作用和巨胞饮作用介导的
最近的证据表明,包括外泌体和微囊泡的细胞外囊泡(EVs)形成了一种内源性运输系统,通过该系统,包括蛋白质和RNA在内的生物分子可以在细胞之间交换。这为电动汽车提供了巨大的潜力,可用于药物输送和再生医学应用。了解基于EV的细胞间转运的生物学基础对于基于EV的治疗剂的开发非常重要。在这里,我们试图表征参与电动汽车摄取的细胞机制。
在HeLa细胞,2D(单层)细胞培养以及3D球体中评估了荧光标记的EV的内在化。使用流式细胞术和共聚焦显微镜,通过化学和基于RNA干扰的抑制作用于单个内吞途径的关键蛋白来评估摄取。化学抑制剂的实验表明,电动汽车的摄取取决于胆固醇和酪氨酸激酶活性,这与网格蛋白非依赖性内吞作用有关,与Na + / H +有关交换和磷酸肌醇3-激酶活性,这对于巨胞饮作用很重要。此外,EV内部化被siRNA介导的小窝蛋白1,弗洛特林1,RhoA,Rac1和PAK1的敲低抑制,但网格蛋白重链没有被抑制。总之,这些结果表明,电动汽车主要通过网格蛋白非依赖性内吞作用和巨胞饮作用进入细胞。鉴定可通过导致功能性货物转移的途径促进其吸收的EV成分,可以通过EV启发的工程技术开发出更有效的治疗方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号