当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Analysis of Enzymes Immobilized in Porous Film Arrays
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acs.analchem.7b02075 Hector D. Neira 1 , Amy E. Herr 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acs.analchem.7b02075 Hector D. Neira 1 , Amy E. Herr 1
Affiliation
|
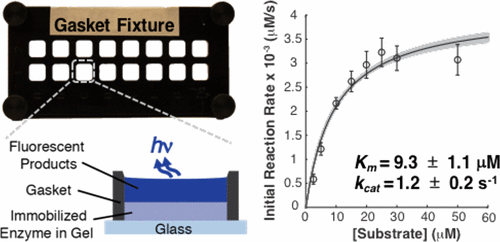
Measuring the catalytic activity of immobilized enzymes underpins development of biosensing, bioprocessing, and analytical chemistry tools. To expand the range of approaches available for measuring enzymatic activity, we report on a technique to probe activity of enzymes immobilized in porous materials in the absence of confounding mass transport artifacts. We measured reaction kinetics of calf intestinal alkaline phosphatase (CIAP) immobilized in benzophenone-modified polyacrylamide (BPMA-PAAm) gel films housed in an array of fluidically isolated chambers. To ensure kinetics measurements are not confounded by mass transport limitations, we employed Weisz’s modulus (Φ), which compares observed enzyme-catalyzed reaction rates to characteristic substrate diffusion times. We characterized activity of CIAP immobilized in BPMA-PAAm gels in a reaction-limited regime (Φ ≪ 0.15 for all measurements), allowing us to isolate the effect of immobilization on enzymatic activity. Immobilization of CIAP in BPMA-PAAm gels produced a ∼2× loss in apparent enzyme–substrate affinity (Km) and ∼200× decrease in intrinsic catalytic activity (kcat) relative to in-solution measurements. As estimating Km and kcat requires multiple steps of data manipulation, we developed a computational approach (bootstrapping) to propagate uncertainty in calibration data through all data manipulation steps. Numerical simulation revealed that calibration error is only negligible when the normalized root-mean-squared error (NRMSE) in the calibration falls below 0.05%. Importantly, bootstrapping is independent of the mathematical model, and thus generalizable beyond enzyme kinetics studies. Furthermore, the measurement tool presented can be readily adapted to study other porous immobilization supports, facilitating rational design (immobilization method, geometry, enzyme loading) of immobilized-enzyme devices.
中文翻译:

固定在多孔膜阵列中的酶的动力学分析
测量固定化酶的催化活性可支持生物传感,生物加工和分析化学工具的发展。为了扩大可用于测量酶活性的方法的范围,我们报告了一种在不存在混淆的传质假象的情况下探测固定在多孔材料中的酶活性的技术。我们测量了固定在一系列流体隔离室中的二苯甲酮改性聚丙烯酰胺(BPMA-PAAm)凝胶膜中固定的小牛肠碱性磷酸酶(CIAP)的反应动力学。为确保动力学测量不受传质限制的困扰,我们采用了韦氏模量(Φ),该模量将观察到的酶催化反应速率与特征底物扩散时间进行了比较。我们以反应受限的方式(在所有测量中,Φ0.15 0.15)表征了固定在BPMA-PAAm凝胶中的CIAP的活性,从而使我们能够分离出固定化对酶活性的影响。将CIAP固定在BPMA-PAAm凝胶中会使表观酶-底物亲和力降低约2倍(K m)和固有催化活性(k cat)相对于溶液中的测量值会降低约200倍。估计K m和k cat需要多个数据操作步骤,我们开发了一种计算方法(自举)来通过所有数据操作步骤传播校准数据中的不确定性。数值模拟表明,仅当校准中的归一化均方根误差(NRMSE)低于0.05%时,校准误差才可以忽略不计。重要的是,自举与数学模型无关,因此可以推广到酶动力学研究之外。此外,所提供的测量工具可以轻松地用于研究其他多孔固定支架,从而有助于固定酶装置的合理设计(固定方法,几何形状,酶负载)。
更新日期:2017-09-15
中文翻译:

固定在多孔膜阵列中的酶的动力学分析
测量固定化酶的催化活性可支持生物传感,生物加工和分析化学工具的发展。为了扩大可用于测量酶活性的方法的范围,我们报告了一种在不存在混淆的传质假象的情况下探测固定在多孔材料中的酶活性的技术。我们测量了固定在一系列流体隔离室中的二苯甲酮改性聚丙烯酰胺(BPMA-PAAm)凝胶膜中固定的小牛肠碱性磷酸酶(CIAP)的反应动力学。为确保动力学测量不受传质限制的困扰,我们采用了韦氏模量(Φ),该模量将观察到的酶催化反应速率与特征底物扩散时间进行了比较。我们以反应受限的方式(在所有测量中,Φ0.15 0.15)表征了固定在BPMA-PAAm凝胶中的CIAP的活性,从而使我们能够分离出固定化对酶活性的影响。将CIAP固定在BPMA-PAAm凝胶中会使表观酶-底物亲和力降低约2倍(K m)和固有催化活性(k cat)相对于溶液中的测量值会降低约200倍。估计K m和k cat需要多个数据操作步骤,我们开发了一种计算方法(自举)来通过所有数据操作步骤传播校准数据中的不确定性。数值模拟表明,仅当校准中的归一化均方根误差(NRMSE)低于0.05%时,校准误差才可以忽略不计。重要的是,自举与数学模型无关,因此可以推广到酶动力学研究之外。此外,所提供的测量工具可以轻松地用于研究其他多孔固定支架,从而有助于固定酶装置的合理设计(固定方法,几何形状,酶负载)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号