当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deciphering the Mechanism of the Nickel-Catalyzed Hydroalkoxylation Reaction: A Combined Experimental and Computational Study
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acscatal.7b00616 Alexis Mifleur 1 , Delphine S. Mérel 1 , André Mortreux 1 , Isabelle Suisse 1 , Frédéric Capet 1 , Xavier Trivelli 2 , Mathieu Sauthier 1 , Stuart A. Macgregor 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acscatal.7b00616 Alexis Mifleur 1 , Delphine S. Mérel 1 , André Mortreux 1 , Isabelle Suisse 1 , Frédéric Capet 1 , Xavier Trivelli 2 , Mathieu Sauthier 1 , Stuart A. Macgregor 3
Affiliation
|
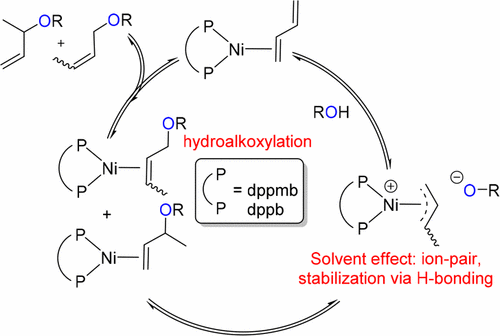
The [Ni(0)(cod)2]/P∩P-catalyzed hydroalkoxylation of butadiene to form butenyl ethers is studied mechanistically, where P∩P = 1,4-bis(diphenylphosphino)butane (dppb) and 1,2-bis(diphenylphosphinomethyl)benzene (dppmb). Experimental studies suggest the intermediacy of [(P∩P)Ni(0)(butadiene)] and [(P∩P)Ni(II)(allyl)] intermediates and rule out the involvement of Ni–H species. The related species [(dppb)Ni(0)(1,4-diphenylbutadiene)], 1, and [(P∩P)Ni(II)(crotyl)(Cl)] complexes 2 (P∩P = dppmb) and 3 (P∩P = dppb) have been synthesized and characterized on the basis of VT NMR spectroscopy and X-ray crystallographic studies. Compounds 2 and 3 are shown to be catalytically competent for the hydroalkoxylation reaction. Computational studies on [(dppmb)Ni(0)(butadiene)] indicate a facile protonation that forms a cationic allylic intermediate [(dppmb)Ni(II)(η-C4H7)]OMe. C–O bond formation then occurs via external attack by the solvent-stabilized methoxide nucleophile. Hydroalkoxylation proceeds with modest computed barriers of ca. 18 kcal/mol, and the butenyl ether product formation is only marginally exergonic. Overall, the results are consistent with initial kinetic control leading to the major branched isomer followed by a reversible isomerization process operating under thermodynamic control.
中文翻译:

镍催化的氢烷氧基化反应机理的解释:结合实验和计算研究。
从机理上研究了[Ni(0)(cod)2 ] / P∩P催化的丁二烯加氢烷氧基化反应生成丁烯基醚,其中P∩P = 1,4-双(二苯基膦基)丁烷(dppb)和1,2-双(二苯基膦基甲基)苯(dppmb)。实验研究表明,[(P∩P)Ni(0)(丁二烯)]和[(PPP)Ni(II)(烯丙基)]中间体具有中介作用,并排除了Ni–H物种的参与。相关物种[(DPPB)镍(0)(1,4-二苯基)] 1和[(P ∩ P)的Ni(II)(巴豆基)(CL)]配合物2(P ∩ P = dppmb)和3(P∩P = dppb)已合成,并根据VT NMR光谱和X射线晶体学研究对其进行了表征。化合物2和3显示出催化加氢烷氧基化反应的能力。上计算的研究[(dppmb)镍(0)(丁二烯)]表示一个浅显的质子化,其形成阳离子烯丙基中间体[(dppmb)镍(II)(η-C 4 H ^ 7)] OMe。然后,由溶剂稳定的甲醇盐亲核试剂通过外部攻击而形成C–O键。加氢烷氧基化以约适度的计算的势垒进行。18 kcal / mol,而丁烯基醚产物的形成仅在很小的程度上是能动的。总体而言,结果与最初的动力学控制相一致,从而导致了主要的支链异构体,随后是在热力学控制下进行的可逆异构化过程。
更新日期:2017-09-15
中文翻译:

镍催化的氢烷氧基化反应机理的解释:结合实验和计算研究。
从机理上研究了[Ni(0)(cod)2 ] / P∩P催化的丁二烯加氢烷氧基化反应生成丁烯基醚,其中P∩P = 1,4-双(二苯基膦基)丁烷(dppb)和1,2-双(二苯基膦基甲基)苯(dppmb)。实验研究表明,[(P∩P)Ni(0)(丁二烯)]和[(PPP)Ni(II)(烯丙基)]中间体具有中介作用,并排除了Ni–H物种的参与。相关物种[(DPPB)镍(0)(1,4-二苯基)] 1和[(P ∩ P)的Ni(II)(巴豆基)(CL)]配合物2(P ∩ P = dppmb)和3(P∩P = dppb)已合成,并根据VT NMR光谱和X射线晶体学研究对其进行了表征。化合物2和3显示出催化加氢烷氧基化反应的能力。上计算的研究[(dppmb)镍(0)(丁二烯)]表示一个浅显的质子化,其形成阳离子烯丙基中间体[(dppmb)镍(II)(η-C 4 H ^ 7)] OMe。然后,由溶剂稳定的甲醇盐亲核试剂通过外部攻击而形成C–O键。加氢烷氧基化以约适度的计算的势垒进行。18 kcal / mol,而丁烯基醚产物的形成仅在很小的程度上是能动的。总体而言,结果与最初的动力学控制相一致,从而导致了主要的支链异构体,随后是在热力学控制下进行的可逆异构化过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号