当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ammonia Synthesis on Wool-Like Au, Pt, Pd, Ag, or Cu Electrode Catalysts in Nonthermal Atmospheric-Pressure Plasma of N2 and H2
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acscatal.7b01624 Masakazu Iwamoto 1 , Mao Akiyama 1 , Keigo Aihara 1 , Takashi Deguchi 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acscatal.7b01624 Masakazu Iwamoto 1 , Mao Akiyama 1 , Keigo Aihara 1 , Takashi Deguchi 1
Affiliation
|
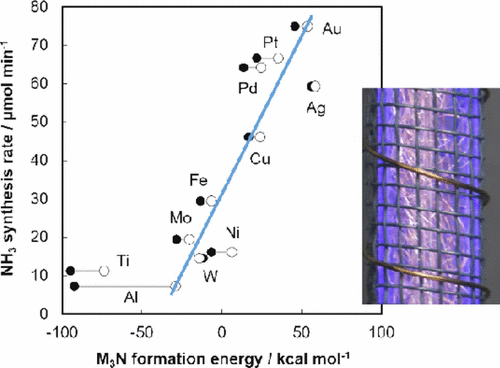
Developing an ammonia synthesis process from N2 and H2 is of interest in the catalysis and hydrogen research community. Wool-like metal electrodes used to produce nonthermal plasma were determined to serve as efficient catalysts for ammonia synthesis under atmospheric pressure without heating. The catalytic activity of Pt, Pd, Ag, Cu, and Ni wools increased as the experiment was repeated, while that of Au, Fe, Mo, Ti, W, and Al was almost constant. The activity change was mainly due to migration of metals from the electrode to the inner wall of a silica reactor or increases in surface areas of metal catalysts. The order of the activity at each initial experiment was Au > Pt > Pd > Ag > Cu > Fe > Mo > Ni > W > Ti > Al. DFT calculations using Gaussian 09 and CASTEP were applied for energy changes in a reaction M3 + 1/2 N2 → M3N and in adsorption of a nitrogen atom on metal surface, in which M3 was a virtual minimum unit of the metal surface. The reactions were assumed to be an essential step in the ammonia production after plasma-activation of N2. The resulting values correlated with the respective initial catalytic activity, indicating that a more unstable M3N surface intermediate produced higher catalytic activity. Emission spectra in the plasma process using various electrodes were measured and showed that the efficiency of electrodes for plasma activation of nitrogen molecules was almost independent of the metals, while the reactivity of the activated species to form ammonia depended greatly on the metal used. The N2/H2 ratio dependence and formation/decomposition rate constants of ammonia were finally determined on Au and Cu, which were different from those for the conventional Haber–Bosch process. The decomposition of produced ammonia was suggested to proceed in a plasma-irradiated gas phase.
中文翻译:

N 2和H 2的非热常压等离子体下的羊毛状Au,Pt,Pd,Ag或Cu电极催化剂上的氨合成
从N 2和H 2开发氨合成工艺在催化和氢气研究界很感兴趣。确定用于产生非热等离子体的羊毛状金属电极可在大气压下不加热的情况下用作合成氨的有效催化剂。重复进行实验,Pt,Pd,Ag,Cu和Ni羊毛的催化活性增加,而Au,Fe,Mo,Ti,W和Al的催化活性几乎恒定。活性变化主要是由于金属从电极向二氧化硅反应器的内壁迁移或金属催化剂的表面积增加。在每个初始实验中,活性的顺序为Au> Pt> Pd> Ag> Cu> Fe> Mo> Ni> W> Ti> Al。N和氮原子在金属表面上的吸附,其中M 3是金属表面的虚拟最小单位。假定该反应是等离子体活化N 2后氨生产中必不可少的步骤。所得值与各自的初始催化活性相关,表明更不稳定的M 3 N表面中间体产生更高的催化活性。测量了在使用各种电极的等离子体过程中的发射光谱,结果表明,电极对氮分子进行等离子体活化的效率几乎与金属无关,而活化物种形成氨的反应性很大程度上取决于所使用的金属。N 2 / H 2最终确定了在Au和Cu上氨的比例依赖性和氨的形成/分解速率常数,这与常规的Haber-Bosch工艺不同。产生的氨的分解被认为是在等离子体辐照的气相中进行的。
更新日期:2017-09-15
中文翻译:

N 2和H 2的非热常压等离子体下的羊毛状Au,Pt,Pd,Ag或Cu电极催化剂上的氨合成
从N 2和H 2开发氨合成工艺在催化和氢气研究界很感兴趣。确定用于产生非热等离子体的羊毛状金属电极可在大气压下不加热的情况下用作合成氨的有效催化剂。重复进行实验,Pt,Pd,Ag,Cu和Ni羊毛的催化活性增加,而Au,Fe,Mo,Ti,W和Al的催化活性几乎恒定。活性变化主要是由于金属从电极向二氧化硅反应器的内壁迁移或金属催化剂的表面积增加。在每个初始实验中,活性的顺序为Au> Pt> Pd> Ag> Cu> Fe> Mo> Ni> W> Ti> Al。N和氮原子在金属表面上的吸附,其中M 3是金属表面的虚拟最小单位。假定该反应是等离子体活化N 2后氨生产中必不可少的步骤。所得值与各自的初始催化活性相关,表明更不稳定的M 3 N表面中间体产生更高的催化活性。测量了在使用各种电极的等离子体过程中的发射光谱,结果表明,电极对氮分子进行等离子体活化的效率几乎与金属无关,而活化物种形成氨的反应性很大程度上取决于所使用的金属。N 2 / H 2最终确定了在Au和Cu上氨的比例依赖性和氨的形成/分解速率常数,这与常规的Haber-Bosch工艺不同。产生的氨的分解被认为是在等离子体辐照的气相中进行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号