当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamics at a Peptide–TiO2 Anatase (101) Interface
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acs.jpcb.7b04707 Marco Polimeni 1 , Loukas Petridis 2 , Jeremy C. Smith 2 , Caterina Arcangeli 3
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acs.jpcb.7b04707 Marco Polimeni 1 , Loukas Petridis 2 , Jeremy C. Smith 2 , Caterina Arcangeli 3
Affiliation
|
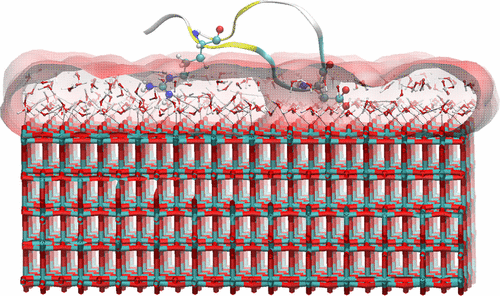
The interface between biological matter and inorganic materials is a widely investigated research topic due to possible applications in biomedicine and nanotechnology. In this context, the molecular level adsorption mechanism that drives specific recognition between small peptide sequences and inorganic surfaces represents an important topic likely to provide much information useful for designing bioderived materials. Here, we investigate the dynamics at the interface between a Ti-binding peptide sequence (AMRKLPDAPGMHC) and a TiO2 anatase surface by using molecular dynamics (MD) simulations. In the simulations the adsorption mechanism is characterized by diffusion of the peptide from the bulk water phase toward the TiO2 surface, followed by the anchoring of the peptide to the surface. The anchoring is mediated by the interfacial water layers by means of the charged groups of the side chains of the peptide. The peptide samples anchored and dissociated states from the surface and its conformation is not affected by the surface when anchored.
中文翻译:

肽-TiO 2锐钛矿(101)界面的动力学
由于在生物医学和纳米技术中的可能应用,生物物质与无机材料之间的界面是一个被广泛研究的研究课题。在这种情况下,驱动小肽序列和无机表面之间特异性识别的分子水平吸附机制代表了一个重要的主题,很可能会提供许多信息,可用于设计生物衍生材料。在这里,我们通过使用分子动力学(MD)模拟研究Ti结合肽序列(AMRKLPDAPGMHC)和TiO 2锐钛矿表面之间的界面动力学。在模拟中,吸附机理的特征是肽从本体水相向TiO 2扩散表面,然后将肽锚定到表面。锚定是通过界面水层借助于肽的侧链的带电基团介导的。肽样品从表面锚定和解离状态,并且其构象在锚定时不受表面影响。
更新日期:2017-09-15
中文翻译:

肽-TiO 2锐钛矿(101)界面的动力学
由于在生物医学和纳米技术中的可能应用,生物物质与无机材料之间的界面是一个被广泛研究的研究课题。在这种情况下,驱动小肽序列和无机表面之间特异性识别的分子水平吸附机制代表了一个重要的主题,很可能会提供许多信息,可用于设计生物衍生材料。在这里,我们通过使用分子动力学(MD)模拟研究Ti结合肽序列(AMRKLPDAPGMHC)和TiO 2锐钛矿表面之间的界面动力学。在模拟中,吸附机理的特征是肽从本体水相向TiO 2扩散表面,然后将肽锚定到表面。锚定是通过界面水层借助于肽的侧链的带电基团介导的。肽样品从表面锚定和解离状态,并且其构象在锚定时不受表面影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号