当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic Requirements of Aldehyde Transfer Hydrogenation Catalyzed by Microporous Solid Brønsted Acid Catalysts
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acscatal.7b01403 Fan Lin 1 , Yifei Yang 1 , Ya-Huei Chin 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acscatal.7b01403 Fan Lin 1 , Yifei Yang 1 , Ya-Huei Chin 1
Affiliation
|
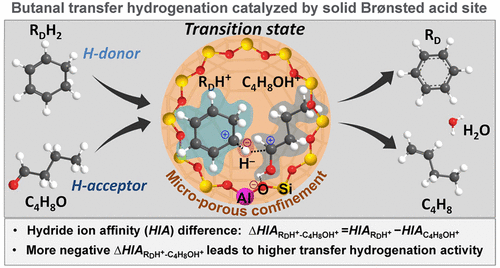
Brønsted acids in a confined environment catalyze transfer hydrogenation of aldehydes (CnH2nO, n = 3–6) by kinetically relevant hydride transfer between hydride donors (e.g., cyclohexadiene, tetralin, cyclohexene, or 3-methyl-1-pentene) and protonated aldehydes. The hydride ion affinity difference between the donor and acceptor pair determines the reaction enthalpy, which reflects the thermodynamic tendency, of hydride transfer. For this class of reactions, the hydride ion affinity difference appears to be the empirical kinetic descriptor for predicting the rates of hydride transfer and, in turn, of transfer hydrogenation through the Brønsted–Evans–Polanyi relation.
中文翻译:

微孔固体布朗斯台德酸催化剂催化醛转移加氢的动力学要求
在密闭环境中,布朗斯台德酸通过氢化物供体(例如环己二烯,四氢萘,环己烯或3-甲基-1-戊烯)之间的动力学相关的氢化物转移催化醛的转移氢化(C n H 2 n O,n = 3–6) )和质子化的醛。供体对与受体对之间的氢化物离子亲和力差异决定了反应焓,反应焓反映了氢化物转移的热力学趋势。对于此类反应,氢化物离子亲和力差异似乎是通过Brønsted–Evans–Polanyi关系预测氢化物转移速率以及反过来转移氢化速率的经验动力学指标。
更新日期:2017-09-14
中文翻译:

微孔固体布朗斯台德酸催化剂催化醛转移加氢的动力学要求
在密闭环境中,布朗斯台德酸通过氢化物供体(例如环己二烯,四氢萘,环己烯或3-甲基-1-戊烯)之间的动力学相关的氢化物转移催化醛的转移氢化(C n H 2 n O,n = 3–6) )和质子化的醛。供体对与受体对之间的氢化物离子亲和力差异决定了反应焓,反应焓反映了氢化物转移的热力学趋势。对于此类反应,氢化物离子亲和力差异似乎是通过Brønsted–Evans–Polanyi关系预测氢化物转移速率以及反过来转移氢化速率的经验动力学指标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号