European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-09-14 , DOI: 10.1016/j.ejmech.2017.09.017 Zeyan Zhang , Xingpeng Xiao , Tong Su , Jinyi Wu , Jianwei Ren , Jiongchang Zhu , Xiaodong Zhang , Rihui Cao , Runlei Du
|
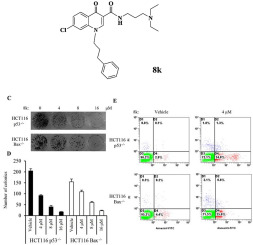
A series of novel water-soluble 4-quinolone-3-carboxamides was prepared and evaluated as antiproliferative agents. Preliminary results indicated that most compounds tested in this study showed potent antiproliferative potencies against human tumor cell lines, and compound 8k was found to be the most potent antiproliferative agents with IC50 value of lower than 10 μM against nine human tumor cell lines. These results suggested that (1) the alkylamino side chain substituent was the advisable pharmacophoric group for the enhanced antiproliferative activities; (2) the length of the alkylamino side chain moiety also affected their antiproliferative potencies, and three methylene units were more favorable; (3) introducing arylated alkyl substituent into N1-position of quinolone facilitated antiproliferative activities of this class of compounds. Further investigations on mechanism of action of this class of compound demonstrated that the representative compound 8k could trigger p53/Bax-independent colorectal cancer cell apoptosis via inducing ROS accumulation.
中文翻译:

新型水溶性4-喹诺酮-3-羧酰胺类抗增殖剂的合成,构效关系及作用的初步机理
制备了一系列新型的水溶性4-喹诺酮-3-羧酰胺,并将其作为抗增殖剂进行了评估。初步结果表明,该研究中测试的大多数化合物对人肿瘤细胞系均显示出有效的抗增殖能力,并且化合物8k被认为是IC 50最有效的抗增殖剂。对9种人类肿瘤细胞株的抗药性值低于10μM。这些结果表明(1)烷基氨基侧链取代基是增强抗增殖活性的可取的药效基团;(2)烷基氨基侧链部分的长度也影响了它们的抗增殖能力,并且三个亚甲基单元更有利;(3)将芳基化的烷基取代基引入喹诺酮的N1-位促进了这类化合物的抗增殖活性。对该化合物作用机理的进一步研究表明,代表性化合物8k可通过诱导ROS积累来触发不依赖p53 / Bax的结直肠癌细胞凋亡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号