Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling Protein Surface Orientation by Strategic Placement of Oligo-Histidine Tags
ACS Nano ( IF 15.8 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acsnano.7b03717 Dorothee Wasserberg , Jordi Cabanas-Danés , Jord Prangsma , Shane O’Mahony 1 , Pierre-Andre Cazade 1 , Eldrich Tromp , Christian Blum , Damien Thompson 1 , Jurriaan Huskens , Vinod Subramaniam 2 , Pascal Jonkheijm
ACS Nano ( IF 15.8 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acsnano.7b03717 Dorothee Wasserberg , Jordi Cabanas-Danés , Jord Prangsma , Shane O’Mahony 1 , Pierre-Andre Cazade 1 , Eldrich Tromp , Christian Blum , Damien Thompson 1 , Jurriaan Huskens , Vinod Subramaniam 2 , Pascal Jonkheijm
Affiliation
|
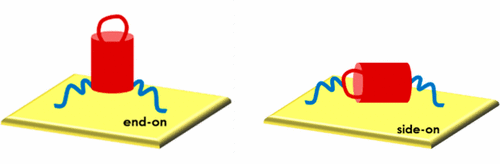
We report oriented immobilization of proteins using the standard hexahistidine (His6)-Ni2+:NTA (nitrilotriacetic acid) methodology, which we systematically tuned to give control of surface coverage. Fluorescence microscopy and surface plasmon resonance measurements of self-assembled monolayers (SAMs) of red fluorescent proteins (TagRFP) showed that binding strength increased by 1 order of magnitude for each additional His6-tag on the TagRFP proteins. All TagRFP variants with His6-tags located on only one side of the barrel-shaped protein yielded a 1.5 times higher surface coverage compared to variants with His6-tags on opposite sides of the so-called β-barrel. Time-resolved fluorescence anisotropy measurements supported by polarized infrared spectroscopy verified that the orientation (and thus coverage and functionality) of proteins on surfaces can be controlled by strategic placement of a His6-tag on the protein. Molecular dynamics simulations show how the differently tagged proteins reside at the surface in “end-on” and “side-on” orientations with each His6-tag contributing to binding. Also, not every dihistidine subunit in a given His6-tag forms a full coordination bond with the Ni2+:NTA SAMs, which varied with the position of the His6-tag on the protein. At equal valency but different tag positions on the protein, differences in binding were caused by probing for Ni2+:NTA moieties and by additional electrostatic interactions between different fractions of the β-barrel structure and charged NTA moieties. Potential of mean force calculations indicate there is no specific single-protein interaction mode that provides a clear preferential surface orientation, suggesting that the experimentally measured preference for the end-on orientation is a supra-protein, not a single-protein, effect.
中文翻译:

通过寡聚组氨酸标签的战略定位来控制蛋白质表面取向。
我们报告了使用标准的六组氨酸(His 6)-Ni 2+:NTA(亚硝酸三乙酸)方法进行定向的蛋白质固定化,我们对其进行了系统地调整以控制表面覆盖率。红色荧光蛋白(TagRFP)自组装单层(SAM)的荧光显微镜和表面等离子体共振测量表明,TagRFP蛋白上每增加一个His 6标签,结合强度都会增加1个数量级。所有带有His 6标签的TagRFP变体仅位于桶形蛋白的一侧,其表面覆盖率比带有His 6的变体高1.5倍所谓的“β-barrel”的相对两侧的标签。极化红外光谱技术支持的时间分辨荧光各向异性测量结果证明,可以通过在蛋白质上进行His 6标签的战略定位来控制蛋白质在表面上的方向(以及覆盖范围和功能性)。分子动力学模拟显示了不同标记的蛋白质如何以“末端”和“侧面”方向停留在表面,而每个His 6标签都有助于结合。同样,并非给定His 6标签中的每个二组氨酸亚基均与Ni 2+:NTA SAMs形成完全配位键,而Ni 2+:NTA SAMs随His 6的位置而变化标签上的蛋白质。在价相同但蛋白质上标签位置不同的情况下,结合的差异是由探测Ni 2+:NTA部分以及β-桶结构的不同部分与带电NTA部分之间的额外静电相互作用引起的。平均力计算的潜力表明,没有提供明显优先表面取向的特定单蛋白相互作用模式,这表明实验测得的末端取向的偏好是超蛋白效应,而不是单蛋白效应。
更新日期:2017-09-14
中文翻译:

通过寡聚组氨酸标签的战略定位来控制蛋白质表面取向。
我们报告了使用标准的六组氨酸(His 6)-Ni 2+:NTA(亚硝酸三乙酸)方法进行定向的蛋白质固定化,我们对其进行了系统地调整以控制表面覆盖率。红色荧光蛋白(TagRFP)自组装单层(SAM)的荧光显微镜和表面等离子体共振测量表明,TagRFP蛋白上每增加一个His 6标签,结合强度都会增加1个数量级。所有带有His 6标签的TagRFP变体仅位于桶形蛋白的一侧,其表面覆盖率比带有His 6的变体高1.5倍所谓的“β-barrel”的相对两侧的标签。极化红外光谱技术支持的时间分辨荧光各向异性测量结果证明,可以通过在蛋白质上进行His 6标签的战略定位来控制蛋白质在表面上的方向(以及覆盖范围和功能性)。分子动力学模拟显示了不同标记的蛋白质如何以“末端”和“侧面”方向停留在表面,而每个His 6标签都有助于结合。同样,并非给定His 6标签中的每个二组氨酸亚基均与Ni 2+:NTA SAMs形成完全配位键,而Ni 2+:NTA SAMs随His 6的位置而变化标签上的蛋白质。在价相同但蛋白质上标签位置不同的情况下,结合的差异是由探测Ni 2+:NTA部分以及β-桶结构的不同部分与带电NTA部分之间的额外静电相互作用引起的。平均力计算的潜力表明,没有提供明显优先表面取向的特定单蛋白相互作用模式,这表明实验测得的末端取向的偏好是超蛋白效应,而不是单蛋白效应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号