当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
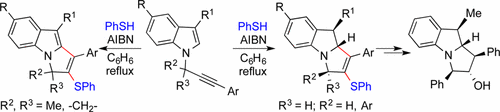
Cascade Radical Cyclization of N-Propargylindoles: Substituents Dictate Stereoselective Formation of N-Fused Indolines versus Indoles
Organic Letters ( IF 5.2 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.orglett.7b02005 Santosh J. Gharpure 1 , Yogesh G. Shelke 1
Organic Letters ( IF 5.2 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.orglett.7b02005 Santosh J. Gharpure 1 , Yogesh G. Shelke 1
Affiliation
|
An efficient protocol for the synthesis of pyrrolo[1,2-a]indole derivatives having sulfide functionality using cascade radical cyclization on propargylindole is described. The nature of the substituents at the propargylic carbon bearing nitrogen of the indole has a profound effect on the rate, yield, and nature of the product obtained by the cascade radical cyclization. An expeditious one-pot route for cascade radical cyclization–desulfurization is also presented. Products obtained were elaborated to the core of the putative structure of the yuremamine and indoline derivative with five contiguous stereocenters.
中文翻译:

N-炔丙酯的级联自由基环化:取代基决定N-稠合二氢吲哚与吲哚的立体选择性形成
描述了在炔丙基吲哚上使用级联自由基环化合成具有硫化物官能团的吡咯并[1,2- a ]吲哚衍生物的有效方案。吲哚的含炔丙基碳的氮上的取代基的性质对级联自由基环化获得的产物的速率,产率和性质具有深远的影响。还提出了一种用于级联自由基环化-脱硫的快速一锅法。将获得的产物精制到具有五个连续立体中心的紫杉醇胺和二氢吲哚衍生物的推定结构的核心。
更新日期:2017-09-13
中文翻译:

N-炔丙酯的级联自由基环化:取代基决定N-稠合二氢吲哚与吲哚的立体选择性形成
描述了在炔丙基吲哚上使用级联自由基环化合成具有硫化物官能团的吡咯并[1,2- a ]吲哚衍生物的有效方案。吲哚的含炔丙基碳的氮上的取代基的性质对级联自由基环化获得的产物的速率,产率和性质具有深远的影响。还提出了一种用于级联自由基环化-脱硫的快速一锅法。将获得的产物精制到具有五个连续立体中心的紫杉醇胺和二氢吲哚衍生物的推定结构的核心。



























 京公网安备 11010802027423号
京公网安备 11010802027423号