当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoselective Double Annulation of Two Different Isocyanides: Rapid Access to Trifluoromethylated Indole-Fused Heterocycles
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.orglett.7b02582 Yuelei Gao 1 , Zhongyan Hu 1 , Jinhuan Dong 2 , Jun Liu 1 , Xianxiu Xu 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.orglett.7b02582 Yuelei Gao 1 , Zhongyan Hu 1 , Jinhuan Dong 2 , Jun Liu 1 , Xianxiu Xu 1, 2
Affiliation
|
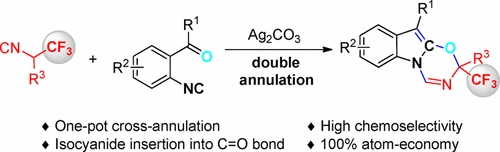
An unprecedented chemoselective double annulation of α-trifluoromethylated isocyanides with o-acylaryl isocyanides has been developed. This new reaction provides a rapid, efficient, and complete atom-economic strategy for the synthesis of trifluoromethylated oxadiazino[3,2-a]indoles in a single operation from readily available starting materials. Isocyanide insertion into C═O double bonds is disclosed for the first time as indicated by the results of 18O-labeling experiment. A mechanism for this domino reaction is proposed involving chemoselective heterodimerization of two different isocyanides, followed by indole-2,3-epoxide formation and rearrangement.
中文翻译:

两种不同异氰酸酯的化学选择性双环:快速获得三氟甲基化的吲哚杂环化合物。
已经开发出空前的α-三氟甲基化异氰酸酯与邻酰基芳基异氰酸酯的化学选择性双环。该新反应提供了快速,有效和完整的原子经济策略,可通过一次操作从容易获得的起始原料合成三氟甲基化的恶二嗪并[3,2- a ]吲哚。由18 O-标记实验的结果表明,异氰酸酯首次插入C = O双键。提出了这种多米诺反应的机制,其中包括两种不同异氰酸酯的化学选择性异二聚化,然后是吲哚-2,3-环氧化物的形成和重排。
更新日期:2017-09-13
中文翻译:

两种不同异氰酸酯的化学选择性双环:快速获得三氟甲基化的吲哚杂环化合物。
已经开发出空前的α-三氟甲基化异氰酸酯与邻酰基芳基异氰酸酯的化学选择性双环。该新反应提供了快速,有效和完整的原子经济策略,可通过一次操作从容易获得的起始原料合成三氟甲基化的恶二嗪并[3,2- a ]吲哚。由18 O-标记实验的结果表明,异氰酸酯首次插入C = O双键。提出了这种多米诺反应的机制,其中包括两种不同异氰酸酯的化学选择性异二聚化,然后是吲哚-2,3-环氧化物的形成和重排。











































 京公网安备 11010802027423号
京公网安备 11010802027423号