Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparison of Carboxybetaine with Sulfobetaine as Lipid Headgroup Involved in Intermolecular Interaction between Lipids in the Membrane
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acsomega.7b00574 Tatsuo Aikawa 1 , Hazuki Okura 1 , Takeshi Kondo 1 , Makoto Yuasa 1
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acsomega.7b00574 Tatsuo Aikawa 1 , Hazuki Okura 1 , Takeshi Kondo 1 , Makoto Yuasa 1
Affiliation
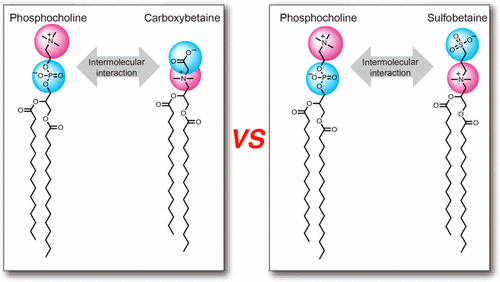
|
Diacylglycerides (DAGs) constitute an important category of lipids owing to their ability to form a lipid membrane, which can be used in a wide variety of biomedical applications. DAGs often include a zwitterionic polar headgroup that can influence the properties of the lipid membrane (e.g., protein adsorption, ion binding, hydration, membrane fluidity, phase stability) and affect their applicability. To clarify the effect of the charge arrangement of zwitterionic headgroups on intermolecular interactions in the DAG bilayers, we investigated the intermolecular interaction between a naturally occurring DAG (1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC)) and synthetic DAGs (which is called “inverse charge zwitterlipids (ICZLs)”) whose headgroup charges were antiparallel with respect to those of DPPC. We used 1,2-dipalmitoyl-sn-glycero-3-carboxybetaine (DPCB) and 1,2-dipalmitoyl-sn-glycero-3-sulfobetaine (DPSB) as ICZLs and compared two combinations of the lipids (DPPC–DPCB and DPPC–DPSB). We obtained surface pressure–area (π–A) isotherms to elucidate the intermolecular interaction between the lipids in the monolayer at the air/water interface. We found shrinkage of the area per molecule in both lipid combinations, indicating that mixing DPPC with ICZLs results in an attractive intermolecular force. As an overall trend, the degree of shrinkage of the mixed monolayer and the thermodynamic favorability of mixing were greater in the DPPC–DPCB combination than in the DPPC–DPSB combination. These trends were also observed in the lipid bilayers, as determined from the gel-to-liquid crystal phase transition temperature (Tc) of the aqueous dispersion of the lipid vesicles. In the highly compressed lipid monolayers and vesicles (lipid bilayer), the molar fractions of ICZLs, in which the intermolecular interaction reached a maximum, were 0.6–0.8 for the DPPC–DPCB combination and 0.5 (equimolar composition) for the DPPC–DPSB combination. Therefore, in the compressed monolayers and bilayers, the mechanism of intermolecular interaction between DPPC and DPCB is different from that between DPPC and DPSB.
中文翻译:

羧基甜菜碱与磺基甜菜碱作为脂质头基参与膜内脂质分子间相互作用的比较
二酰基甘油酯(DAG)因其形成脂质膜的能力而成为一类重要的脂质,可用于多种生物医学应用。DAG 通常包含两性离子极性头基,可以影响脂质膜的特性(例如蛋白质吸附、离子结合、水合、膜流动性、相稳定性)并影响其适用性。为了阐明两性离子头基的电荷排列对 DAG 双层分子间相互作用的影响,我们研究了天然存在的 DAG(1,2-二棕榈酰-sn-甘油-3-磷酸胆碱(DPPC))和合成 DAG 之间的分子间相互作用(称为“反电荷两性脂质(ICZL)”),其头基电荷与 DPPC 的头基电荷反平行。我们使用 1,2-二棕榈酰- sn -甘油-3-羧基甜菜碱 (DPCB) 和 1,2-二棕榈酰- sn -甘油-3-磺基甜菜碱 (DPSB) 作为 ICZL,并比较了两种脂质组合(DPPC-DPCB 和 DPPC) –DPSB)。我们获得了表面压力-面积 (π- A ) 等温线,以阐明空气/水界面单层脂质之间的分子间相互作用。我们发现两种脂质组合中每个分子的面积都有所缩小,这表明将 DPPC 与 ICZL 混合会产生分子间吸引力。总体趋势是,DPPC-DPCB 组合中混合单层的收缩程度和混合的热力学有利性大于 DPPC-DPSB 组合。这些趋势也在脂质双层中观察到,根据脂质囊泡水分散体的凝胶到液晶相变温度(T c )测定。在高度压缩的脂质单层和囊泡(脂质双层)中,分子间相互作用达到最大的ICZL的摩尔分数,DPPC-DPCB组合为0.6-0.8,DPPC-DPSB组合为0.5(等摩尔组成) 。因此,在压缩的单层和双层中,DPPC和DPCB之间的分子间相互作用机制不同于DPPC和DPSB之间的分子间相互作用机制。
更新日期:2017-09-14
中文翻译:

羧基甜菜碱与磺基甜菜碱作为脂质头基参与膜内脂质分子间相互作用的比较
二酰基甘油酯(DAG)因其形成脂质膜的能力而成为一类重要的脂质,可用于多种生物医学应用。DAG 通常包含两性离子极性头基,可以影响脂质膜的特性(例如蛋白质吸附、离子结合、水合、膜流动性、相稳定性)并影响其适用性。为了阐明两性离子头基的电荷排列对 DAG 双层分子间相互作用的影响,我们研究了天然存在的 DAG(1,2-二棕榈酰-sn-甘油-3-磷酸胆碱(DPPC))和合成 DAG 之间的分子间相互作用(称为“反电荷两性脂质(ICZL)”),其头基电荷与 DPPC 的头基电荷反平行。我们使用 1,2-二棕榈酰- sn -甘油-3-羧基甜菜碱 (DPCB) 和 1,2-二棕榈酰- sn -甘油-3-磺基甜菜碱 (DPSB) 作为 ICZL,并比较了两种脂质组合(DPPC-DPCB 和 DPPC) –DPSB)。我们获得了表面压力-面积 (π- A ) 等温线,以阐明空气/水界面单层脂质之间的分子间相互作用。我们发现两种脂质组合中每个分子的面积都有所缩小,这表明将 DPPC 与 ICZL 混合会产生分子间吸引力。总体趋势是,DPPC-DPCB 组合中混合单层的收缩程度和混合的热力学有利性大于 DPPC-DPSB 组合。这些趋势也在脂质双层中观察到,根据脂质囊泡水分散体的凝胶到液晶相变温度(T c )测定。在高度压缩的脂质单层和囊泡(脂质双层)中,分子间相互作用达到最大的ICZL的摩尔分数,DPPC-DPCB组合为0.6-0.8,DPPC-DPSB组合为0.5(等摩尔组成) 。因此,在压缩的单层和双层中,DPPC和DPCB之间的分子间相互作用机制不同于DPPC和DPSB之间的分子间相互作用机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号