当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analysis of the Nucleus-Independent Chemical Shifts of [10]Cyclophenacene: Is It an Aromatic or Antiaromatic Molecule?
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acs.jpclett.7b01937 Guglielmo Monaco 1 , Riccardo Zanasi 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acs.jpclett.7b01937 Guglielmo Monaco 1 , Riccardo Zanasi 1
Affiliation
|
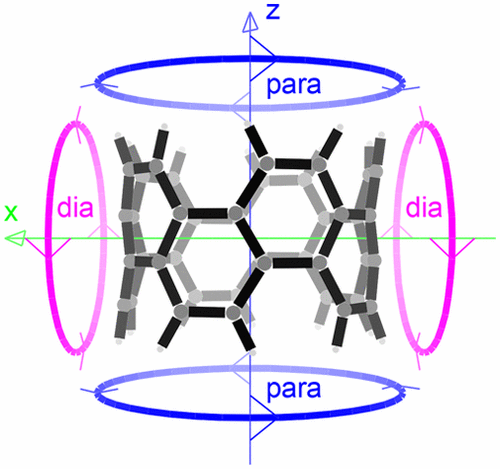
[10]Cyclophenacene is an important synthetic target that shows a pair of nucleus-independent chemical shift (NICS) values for the center of mass and six-membered rings typical of an aromatic species. This is found in contrast with the global paratropic current density induced by a magnetic field parallel to the main symmetry axis. This apparent contradiction has been analyzed by studying the tensor character of the magnetic response. It turns out that the molecule displays two characters, one paratropic (antiaromatic) and another one diatropic (aromatic), depending on the orientation of the inducing magnetic field. The paratropic response, which cannot be recognized from the NICS values, is associated with a well-defined destabilization of the belt closure, as witnessed by homodesmotic reactions. A scalar measure of magnetic aromaticity, the field-independent current strength, has been introduced, which allows us to reach the conclusion that [10]cyclophenacene is indeed an aromatic molecule, although it is significantly affected by the paratropic response.
中文翻译:

[10]并苯的核独立化学位移分析:是芳香族分子还是抗芳香族分子?
[10]并苯是一个重要的合成目标,它显示出一对不依赖于核的化学位移(NICS)值,该值代表质心和典型的芳香族六元环。这与平行于主对称轴的磁场感应的整体副热带电流密度相反。通过研究磁响应的张量特性,分析了这种明显的矛盾。事实证明,根据感应磁场的方向,分子显示出两个字符,一个是顺磁性的(抗芳香族),另一个是一个变质的(芳香族)。从NICS值无法识别的副热响应与带闭合的明确定义的失稳相关,如同渗反应所证明的那样。磁性芳香度的标量度量,
更新日期:2017-09-14
中文翻译:

[10]并苯的核独立化学位移分析:是芳香族分子还是抗芳香族分子?
[10]并苯是一个重要的合成目标,它显示出一对不依赖于核的化学位移(NICS)值,该值代表质心和典型的芳香族六元环。这与平行于主对称轴的磁场感应的整体副热带电流密度相反。通过研究磁响应的张量特性,分析了这种明显的矛盾。事实证明,根据感应磁场的方向,分子显示出两个字符,一个是顺磁性的(抗芳香族),另一个是一个变质的(芳香族)。从NICS值无法识别的副热响应与带闭合的明确定义的失稳相关,如同渗反应所证明的那样。磁性芳香度的标量度量,











































 京公网安备 11010802027423号
京公网安备 11010802027423号