JAMA Oncology ( IF 22.5 ) Pub Date : 2017-09-01 , DOI: 10.1001/jamaoncol.2017.0076 Naomi B Haas 1 , Judith Manola 2 , Janice P Dutcher 3 , Keith T Flaherty 4 , Robert G Uzzo 5 , Michael B Atkins 6 , Robert S DiPaola 7, 8 , Toni K Choueiri 2
|
Importance Given recently published results of a 750-patient adjuvant sunitinib trial showing improved disease-free survival (DFS), the appropriate strategy for treating high-risk patients is unclear. We sought to determine whether there is improved disease-free survival benefit to taking the active drug in patients with high-risk (pT3, pT4, node-positive) clear cell renal cancer (ccRCC) in the ASSURE trial (adjuvant sunitinib or sorafenib vs placebo in resected unfavorable renal cell carcinoma [RCC]), the largest adjuvant trial published to date.
Objective To evaluate DFS and overall survival (OS) in ccRCC high-risk patients randomized to sunitinib or sorafenib vs placebo among patients with stages comparable to other high-risk adjuvant trials.
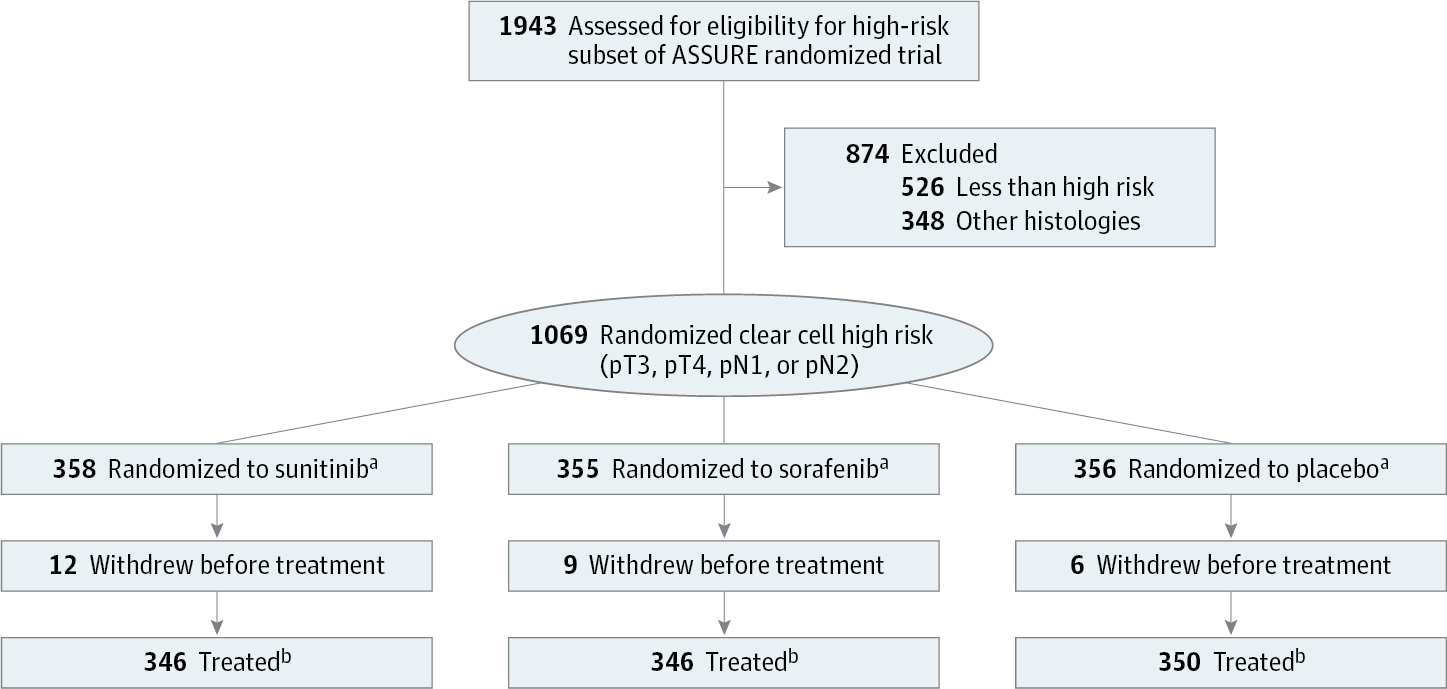
Design, Setting, and Participants The DFS and OS at 10 years postactivation were calculated for 1069 patients in US and Canadian cooperative groups with high-risk patients who had ccRCC histology and pT3, pT4, or node-positive disease accrued between 2006 and 2010 to the double-blind randomized placebo-controlled phase 3 trial. Outcome analyses by dose quartiles of these patients receiving sunitinib or sorafenib were also performed.
Interventions Patients received 1 year of adjuvant sunitinib (50 mg), sorafenib (800 mg) daily, or equivalent placebo. The study was amended for patient intolerance to sunitinib (37.5 mg), sorafenib (400 mg) daily, or equivalent placebo with mandatory dose escalation if no serious adverse effects were experienced.
Main Outcomes and Measures Disease-free survival, defined as time from randomization to recurrence, second primary cancer, or death.
Results Of 1069 patients, 358 (243 [67.9%] men, 115 [32.1%] women) received sunitinib, 355 (248 [69.9%] men, 107 [30.1%] women) received sorafenib, and 356 (254 [71.3%] men, 102 [28.7%] women) received placebo as adjuvant therapy. The mean (SD) age for each group was 58.3 (10.6) years, 56.8 (10.3) years, and 57.5 (10.4) years, respectively. Five-year DFS rates were 47.7%, 49.9%, and 50.0%, respectively for sunitinib, sorafenib, and placebo (HR, 0.94 for sunitinib vs placebo; and HR, 0.90; 97.5% CI, 0.71-1.14 for sorafenib vs placebo), with 5-year OS of 75.2%, 80.2%, and 76.5% (HR, 1.06; 97.5% CI, 0.78-1.45; P = .66, sunitinib vs placebo; and HR, 0.80; 97.5% CI, 0.58-1.11; P = .12 for sorafenib vs placebo). There was no difference by dose quartile.
Conclusions and Relevance Neither prognostic category of the tumor nor dose intensity of therapy altered the lack of difference in DFS or OS in this population of patients with high-risk ccRCC.
Trial Registration clinicaltrials.gov Identifier: NCT00326898
中文翻译:

高风险透明细胞肾癌的辅助治疗更新了 ASSURE 随机试验高风险子集的结果
重要性 鉴于最近公布的一项 750 名患者的辅助舒尼替尼试验结果显示无病生存期 (DFS) 有所改善,因此尚不清楚治疗高危患者的适当策略。我们试图确定在 ASSURE 试验(舒尼替尼或索拉非尼辅助治疗 vs.安慰剂用于切除的不利肾细胞癌 [RCC]),这是迄今为止发表的最大的辅助试验。
目的 评估 ccRCC 高危患者随机接受舒尼替尼或索拉非尼 vs 安慰剂的 DFS 和总生存期 (OS),这些患者的分期与其他高危辅助试验相当。
设计、设置和参与者 计算美国和加拿大合作组中 1069 名患者在激活后 10 年的 DFS 和 OS,这些患者有 ccRCC 组织学和 2006 年至 2010 年间累积的 pT3、pT4 或淋巴结阳性疾病的高危患者双盲随机安慰剂对照 3 期试验。还对这些接受舒尼替尼或索拉非尼的患者按剂量四分位数进行了结果分析。
干预 患者每天接受 1 年的辅助舒尼替尼(50 毫克)、索拉非尼(800 毫克)或等效安慰剂。该研究针对患者对舒尼替尼(37.5 毫克)、索拉非尼(400 毫克)每日或等效安慰剂的不耐受进行了修正,如果没有出现严重的不良反应,则强制增加剂量。
主要结果和措施 无病生存期,定义为从随机化到复发、第二原发癌或死亡的时间。
结果 1069 例患者中,358 例(243 例 [67.9%] 男性,115 例 [32.1%] 女性)接受舒尼替尼治疗,355 例(248 例 [69.9%] 男性,107 例 [30.1%] 女性)接受索拉非尼治疗,356 例(254 [71.3%] ] 男性,102 名 [28.7%] 女性)接受安慰剂作为辅助治疗。每组的平均 (SD) 年龄分别为 58.3 (10.6) 岁、56.8 (10.3) 岁和 57.5 (10.4) 岁。舒尼替尼、索拉非尼和安慰剂的 5 年 DFS 率分别为 47.7%、49.9% 和 50.0%(舒尼替尼对比安慰剂的 HR,0.94;和 HR,0.90;97.5% CI,0.71-1.14 索拉非尼对比安慰剂) ,5 年 OS 分别为 75.2%、80.2% 和 76.5%(HR,1.06;97.5% CI,0.78-1.45;P = .66,舒尼替尼 vs 安慰剂;HR,0.80;97.5% CI,0.58-1.11 ; 索拉非尼与安慰剂的P = .12)。剂量四分位数没有差异。
结论和相关性 肿瘤的预后类别和治疗的剂量强度都没有改变这一高危 ccRCC 患者人群中 DFS 或 OS 的差异。
试验注册 临床试验.gov 标识符:NCT00326898











































 京公网安备 11010802027423号
京公网安备 11010802027423号