JAMA Oncology ( IF 22.5 ) Pub Date : 2017-09-01 , DOI: 10.1001/jamaoncol.2017.0515 Salah-Eddin Al-Batran 1 , Nils Homann 2 , Claudia Pauligk 1 , Gerald Illerhaus 3, 4 , Uwe M Martens 5 , Jan Stoehlmacher 6, 7 , Harald Schmalenberg 8, 9 , Kim B Luley 10 , Nicole Prasnikar 11, 12 , Matthias Egger 13 , Stephan Probst 14 , Helmut Messmann 15 , Markus Moehler 16 , Wolfgang Fischbach 17 , Jörg T Hartmann 18, 19 , Frank Mayer 18, 20 , Heinz-Gert Höffkes 21 , Michael Koenigsmann 22 , Dirk Arnold 23, 24 , Thomas W Kraus 25 , Kersten Grimm 25 , Stefan Berkhoff 25 , Stefan Post 26 , Elke Jäger 27 , Wolf Bechstein 28 , Ulrich Ronellenfitsch 26 , Stefan Mönig 29 , Ralf D Hofheinz 26
|
Importance Surgical resection has a potential benefit for patients with metastatic adenocarcinoma of the stomach and gastroesophageal junction.
Objective To evaluate outcome in patients with limited metastatic disease who receive chemotherapy first and proceed to surgical resection.
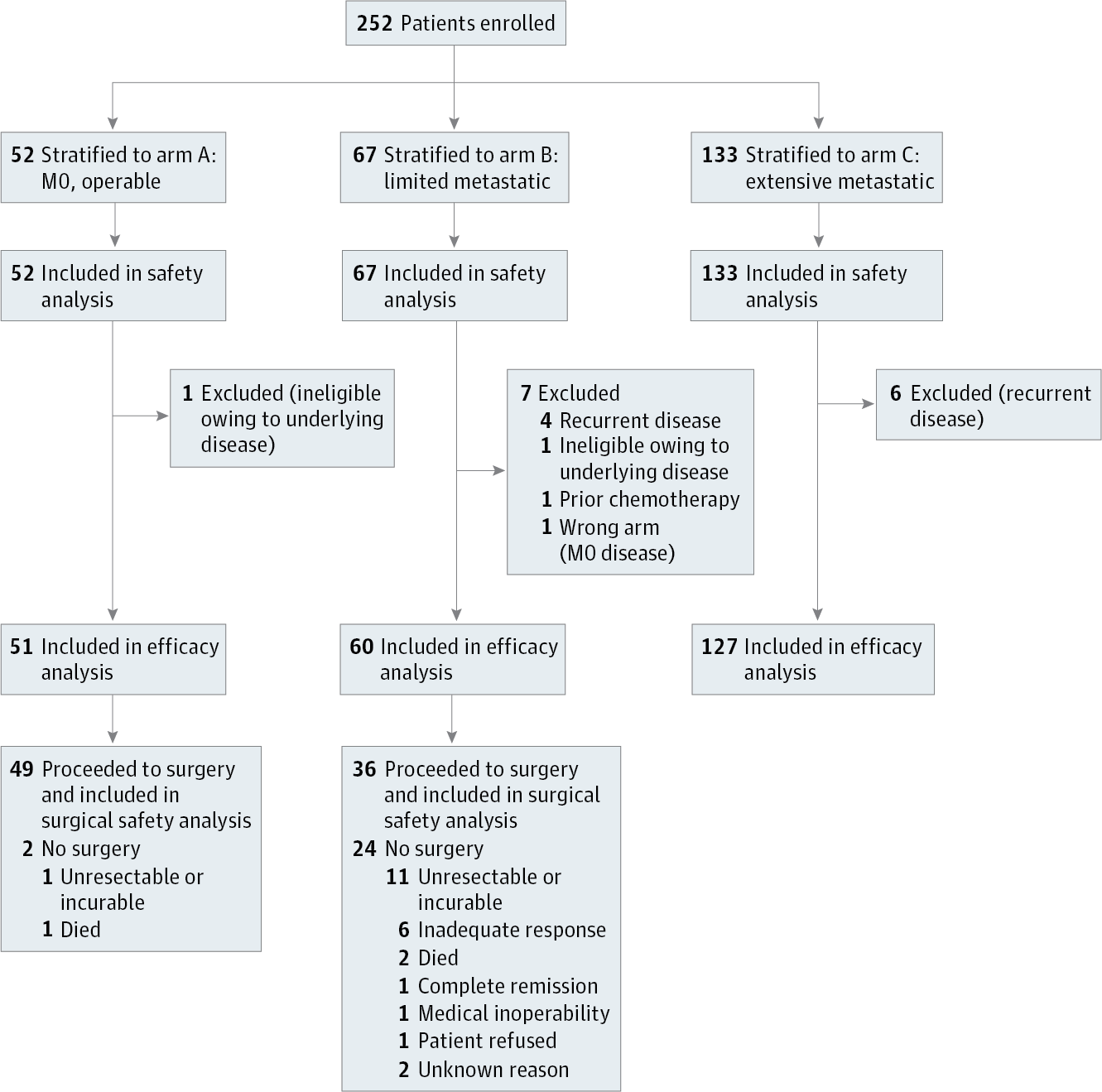
Design, Setting, and Participants The AIO-FLOT3 (Arbeitsgemeinschaft Internistische Onkologie–fluorouracil, leucovorin, oxaliplatin, and docetaxel) trial is a prospective, phase 2 trial of 252 patients with resectable or metastatic gastric or gastroesophageal junction adenocarcinoma. Patients were enrolled from 52 cancer care centers in Germany between February 1, 2009, and January 31, 2010, and stratified to 1 of 3 groups: resectable (arm A), limited metastatic (arm B), or extensive metastatic (arm C). Data cutoff was January 2012, and the analysis was performed in March 2013.
Interventions Patients in arm A received 4 preoperative cycles of fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) followed by surgery and 4 postoperative cycles. Patients in arm B received at least 4 cycles of neoadjuvant FLOT and proceeded to surgical resection if restaging (using computed tomography and magnetic resonance imaging) showed a chance of margin-free (R0) resection of the primary tumor and at least a macroscopic complete resection of the metastatic lesions. Patients in arm C were offered FLOT chemotherapy and surgery only if required for palliation. Patients received a median (range) of 8 (1-15) cycles of FLOT.
Main Outcomes and Measures The primary end point was overall survival.
Results In total, 238 of 252 patients (94.4%) were eligible to participate. The median (range) age of participants was 66 (36-79) years in arm A (n = 51), 63 (28-79) years in arm B (n = 60), and 65 (23-83) years in arm C (n = 127). Patients in arm B (n = 60) had only retroperitoneal lymph node involvement (27 patients [45%]), liver involvement (11 [18.3%]), lung involvement (10 [16.7%]), localized peritoneal involvement (4 [6.7%]), or other (8 [13.3%]) incurable sites. Median overall survival was 22.9 months (95% CI, 16.5 to upper level not achieved) for arm B, compared with 10.7 months (95% CI, 9.1-12.8) for arm C (hazard ratio, 0.37; 95% CI, 0.25-0.55) (P < .001). The response rate for arm B was 60% (complete, 10%; partial, 50%), which is higher than the 43.3% for arm C. In arm B, 36 of 60 patients (60%) proceeded to surgery. The median overall survival was 31.3 months (95% CI, 18.9-upper level not achieved) for patients who proceeded to surgery and 15.9 months (95% CI, 7.1-22.9) for the other patients.
Conclusions and Relevance Patients with limited metastatic disease who received neoadjuvant chemotherapy and proceeded to surgery showed a favorable survival. The AIO-FLOT3 trial provides a rationale for further randomized clinical trials.
Trial Registration clinicaltrials.gov identifier: NCT00849615











































 京公网安备 11010802027423号
京公网安备 11010802027423号