当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design of Disaccharide Modules for a Programmable One‐Pot Synthesis of Building Blocks with LacNAc Repeating Units for Asymmetric N‐Glycans
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-09-14 , DOI: 10.1002/ajoc.201700393 Cheng-Yueh Ting,Yu-Wei Lin,Chung-Yi Wu,Chi-Huey Wong
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-09-14 , DOI: 10.1002/ajoc.201700393 Cheng-Yueh Ting,Yu-Wei Lin,Chung-Yi Wu,Chi-Huey Wong
|
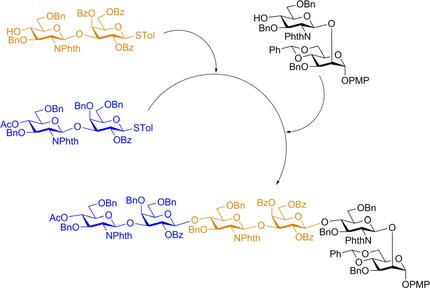
Poly‐N‐acetyllactosamines (poly‐LacNAc) containing the repeating unit of N‐acetyllactosamine (LacNAc, Gal‐β‐1, 4‐GlcNAc) are a general glycan epitope of glycoconjugates that are often sialylated or fucosylated to exhibit significant biological functions. To explore the biological significance of poly‐LacNAc, scientists have made efforts in preparing multi‐antennary N‐glycans linked with different numbers of LacNAc units via chemical or chemoenzymatic approaches. However, current methods have not met the challenge of producing asymmetric N‐glycans with poly‐LacNAc extension for further glycosylation. We have developed a strategy to address this issue using a combined programmable one‐pot synthesis and enzymatic strategy based on glycosyltransferases to prepare linear poly‐LacNAc and derivatives. The one‐pot synthesis is carried out with the use of building blocks that have the “GlcNAc‐β‐(1, 3)‐Gal” skeleton. The LacNAc repeating units can be deprotected and further glycosylated enzymatically, as illustrated by the galactosyl transferase and α‐2, 3‐sialyltransferase reactions, to add galactose and sialic acid sequentially, to reduce the complexity of protecting group manipulation. In addition, the synthetic LacNAc derivatives contain a temporary PMP protecting group at the anomeric position that can be easily removed and converted into an oligosaccharyl donor such as glycosyl fluoride for the subsequent synthesis of asymmetric N‐glycans.
中文翻译:

用于不对称N-聚糖的LacNAc重复单元的可编程单罐合成砌块的二糖模块设计
聚Ñ -acetyllactosamines(聚的LacNAc)含有的重复单元Ñ乙酰乙酰乳糖胺(LacNAc,Gal-β-1、4-GlcNAc)是糖缀合物的一般聚糖表位,通常被唾液酸化或岩藻糖基化,表现出重要的生物学功能。为了探索poly-LacNAc的生物学意义,科学家们已经努力通过化学或化学酶方法制备与不同数量的LacNAc单位连接的多天线N-聚糖。然而,目前的方法还没有解决产生具有多聚LacNAc延伸的不对称N-聚糖以进一步糖基化的挑战。我们已开发出一种策略来解决此问题,它使用结合的基于糖基转移酶的可编程单锅合成和酶促策略制备线性poly-LacNAc及其衍生物。使用具有“GlcNAc-β-(1,3)-Gal”骨架的构件进行一锅法合成。如半乳糖基转移酶和α-2,3-唾液酸转移酶反应所示,可以将LacNAc重复单元去保护并进一步酶促糖基化,以依次添加半乳糖和唾液酸,以降低保护基操纵的复杂性。此外,合成的LacNAc衍生物在异头位置含有一个临时PMP保护基,可以轻松除去该保护基并将其转化为寡糖供体,例如糖基氟化物,用于随后的不对称N-聚糖的合成。
更新日期:2017-09-14
中文翻译:

用于不对称N-聚糖的LacNAc重复单元的可编程单罐合成砌块的二糖模块设计
聚Ñ -acetyllactosamines(聚的LacNAc)含有的重复单元Ñ乙酰乙酰乳糖胺(LacNAc,Gal-β-1、4-GlcNAc)是糖缀合物的一般聚糖表位,通常被唾液酸化或岩藻糖基化,表现出重要的生物学功能。为了探索poly-LacNAc的生物学意义,科学家们已经努力通过化学或化学酶方法制备与不同数量的LacNAc单位连接的多天线N-聚糖。然而,目前的方法还没有解决产生具有多聚LacNAc延伸的不对称N-聚糖以进一步糖基化的挑战。我们已开发出一种策略来解决此问题,它使用结合的基于糖基转移酶的可编程单锅合成和酶促策略制备线性poly-LacNAc及其衍生物。使用具有“GlcNAc-β-(1,3)-Gal”骨架的构件进行一锅法合成。如半乳糖基转移酶和α-2,3-唾液酸转移酶反应所示,可以将LacNAc重复单元去保护并进一步酶促糖基化,以依次添加半乳糖和唾液酸,以降低保护基操纵的复杂性。此外,合成的LacNAc衍生物在异头位置含有一个临时PMP保护基,可以轻松除去该保护基并将其转化为寡糖供体,例如糖基氟化物,用于随后的不对称N-聚糖的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号