Catalysis Today ( IF 5.2 ) Pub Date : 2017-09-14 , DOI: 10.1016/j.cattod.2017.09.013 Said Said Abubakar , Maurizio Benaglia , Sergio Rossi , Rita Annunziata
|
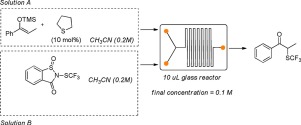
This work describes the organocatalytic α-trifluoromethylthiolation of silylenol ethers using N-(trifluoromethylthio)saccharin as trifluoromethylthiolating reagent that is activated by the presence of catalytic amounts of a Lewis base. Tetrahydrothiophene was identified as the best organocatalyst and it was successfully employed to promote the synthesis of different α-trifluoromethylketones; the reaction has been performed under a traditional batch methodology and under continuous flow conditions. In general, yields obtained using the traditional batch process were higher than those observed when the reaction was performed under flow conditions. However, short reaction times, higher productivity and higher space time yields were observed when a flow system process was employed. Preliminary DFT calculations were also performed in order to elucidate the mechanism of the reaction.
中文翻译:

甲硅烷基醚的有机催化α-三氟甲基硫醇化:间歇与连续流动反应
这项工作描述了使用N的甲硅烷基醚的有机催化α-三氟甲基硫醇化-(三氟甲硫基)糖精作为三氟甲硫基化试剂,可通过催化量的路易斯碱的存在而活化。四氢噻吩被认为是最好的有机催化剂,并成功地用于促进不同α-三氟甲基酮的合成。该反应是在传统的间歇方法和连续流动条件下进行的。通常,使用传统的分批工艺获得的产率高于在流动条件下进行反应时观察到的产率。然而,当采用流动系统工艺时,观察到较短的反应时间,较高的生产率和较高的时空产率。为了阐明反应机理,还进行了初步的DFT计算。











































 京公网安备 11010802027423号
京公网安备 11010802027423号