当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced biofilm penetration for microbial control by polyvalent phages conjugated with magnetic colloidal nanoparticle clusters (CNCs)
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1039/c7en00414a Ling-Li Li 1, 2, 3, 4 , Pingfeng Yu 5, 6, 7, 8 , Xifan Wang 5, 6, 7, 8 , Sheng-Song Yu 1, 2, 3, 4 , Jacques Mathieu 5, 6, 7, 8 , Han-Qing Yu 1, 2, 3, 4 , Pedro J. J. Alvarez 5, 6, 7, 8
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1039/c7en00414a Ling-Li Li 1, 2, 3, 4 , Pingfeng Yu 5, 6, 7, 8 , Xifan Wang 5, 6, 7, 8 , Sheng-Song Yu 1, 2, 3, 4 , Jacques Mathieu 5, 6, 7, 8 , Han-Qing Yu 1, 2, 3, 4 , Pedro J. J. Alvarez 5, 6, 7, 8
Affiliation
|
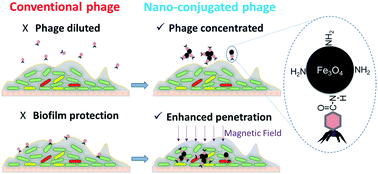
Biofilms may shelter pathogenic or other problematic microorganisms that are difficult to eradicate due to hindered penetration of antimicrobial chemicals. Here, we demonstrate the potential for efficient bacterial suppression using polyvalent (broad host-range) phages attached to magnetic colloidal nanoparticle clusters (CNCs) that facilitate biofilm penetration under a relatively small magnetic field (660 gauss). The polyvalent phage PEL1 (Podoviridae family) was immobilized onto Fe3O4-based magnetic CNCs that had been coated with chitosan (and thus functionalized with amino groups). This facilitated conjugation with phages via covalent bonding (i.e., amide linkages) and enabled phage loading, which reached (5.2 ± 0.7) × 103 centers of infection per 1 μg of chitosan-coated CNCs (CS-Fe3O4). The plaque formation capability of PEL1–CS-Fe3O4 on Pseudomonas aeruginosa PA01 and Escherichia coli C3000 lawns was significantly higher than that of phages conjugated with similar CNCs that had been functionalized with carboxyl groups (99.1% vs. 3.2% Petri dish area of infection). In newly established biofilms formed from these two species on a glass surface, PEL1–CS-Fe3O4 removed 88.7 ± 2.8% of the biofilm coverage area after 6 h of treatment. Overall, this conjugation approach could extend the application of phages for microbial control by enhancing their delivery to relatively inaccessible locations within biofilms.
中文翻译:

与磁性胶体纳米颗粒簇(CNC)共轭的多价噬菌体增强了生物膜渗透性,可用于微生物控制。
生物膜可能掩盖了由于抗微生物化学物质的渗透受阻而难以根除的病原性或其他有问题的微生物。在这里,我们证明了使用附着在磁性胶体纳米颗粒簇(CNC)上的多价(宽宿主范围)噬菌体进行高效细菌抑制的潜力,该噬菌体有助于在相对较小的磁场(660高斯)下穿透生物膜。将多价噬菌体PEL1(Podoviridae家族)固定在已涂有壳聚糖(并因此被氨基官能化)的基于Fe 3 O 4的磁性CNC上。这有助于通过共价键与噬菌体结合(即,酰胺键)和噬菌体负载量(每1μg壳聚糖包被的CNCs(CS-Fe 3 O 4)达到(5.2±0.7)×10 3个感染中心)。在铜绿假单胞菌PA01和大肠杆菌C3000草坪上,PEL1-CS-Fe 3 O 4的噬菌斑形成能力显着高于与已被羧基官能化的类似CNC结合的噬菌体(99.1%vs. 3.2%培养皿面积)感染)。在这两种物质在玻璃表面上新建立的生物膜中,PEL1-CS-Fe 3 O 4处理6小时后,去除了88.7±2.8%的生物膜覆盖面积。总体而言,这种缀合方法可以通过增强噬菌体向生物膜内相对难以接近的位置的递送来扩展其在微生物控制中的应用。
更新日期:2017-09-14
中文翻译:

与磁性胶体纳米颗粒簇(CNC)共轭的多价噬菌体增强了生物膜渗透性,可用于微生物控制。
生物膜可能掩盖了由于抗微生物化学物质的渗透受阻而难以根除的病原性或其他有问题的微生物。在这里,我们证明了使用附着在磁性胶体纳米颗粒簇(CNC)上的多价(宽宿主范围)噬菌体进行高效细菌抑制的潜力,该噬菌体有助于在相对较小的磁场(660高斯)下穿透生物膜。将多价噬菌体PEL1(Podoviridae家族)固定在已涂有壳聚糖(并因此被氨基官能化)的基于Fe 3 O 4的磁性CNC上。这有助于通过共价键与噬菌体结合(即,酰胺键)和噬菌体负载量(每1μg壳聚糖包被的CNCs(CS-Fe 3 O 4)达到(5.2±0.7)×10 3个感染中心)。在铜绿假单胞菌PA01和大肠杆菌C3000草坪上,PEL1-CS-Fe 3 O 4的噬菌斑形成能力显着高于与已被羧基官能化的类似CNC结合的噬菌体(99.1%vs. 3.2%培养皿面积)感染)。在这两种物质在玻璃表面上新建立的生物膜中,PEL1-CS-Fe 3 O 4处理6小时后,去除了88.7±2.8%的生物膜覆盖面积。总体而言,这种缀合方法可以通过增强噬菌体向生物膜内相对难以接近的位置的递送来扩展其在微生物控制中的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号