Particuology ( IF 4.1 ) Pub Date : 2017-06-23 , DOI: 10.1016/j.partic.2017.01.005 Bo Yuan , Jing Wang , Wei Cai , Yurong Yang , Meigui Yi , Lan Xiang
|
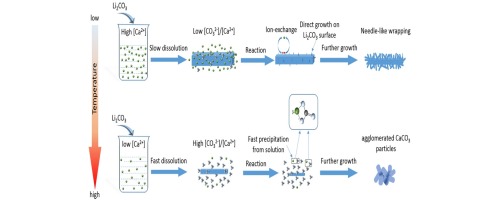
The effects of temperature on the conversion of Li2CO3 to LiOH in a Ca(OH)2 suspension were investigated. Li2CO3 microplates were used as the Li source. The results showed that Li2CO3 was converted to LiOH via in situ ion-exchange and dissolution–precipitation routes. The formation of mixed CaxLi2−2xCO3 intermediate species confirmed that at 25 °C needle-like CaCO3 was formed heterogeneously on the Li2CO3 surface via in situ ion-exchange. At 60–100 °C, isolated CaCO3 agglomerates were formed homogeneously via dissolution–precipitation. Temperature increases accelerated the dissolution and conversion of Li2CO3 to LiOH, producing solutions with high [CO32−]/[Ca2+] ratios; this favored homogeneous precipitation of isolated CaCO3 agglomerates.
中文翻译:

温度对Ca(OH)2悬浮液中Li 2 CO 3转化为LiOH的影响
研究了温度对Ca(OH)2悬浮液中Li 2 CO 3转化为LiOH的影响。Li 2 CO 3微孔板用作Li源。结果表明,Li 2 CO 3通过原位离子交换和溶解-沉淀途径转化为LiOH。混合的Ca x Li 2−2 x CO 3中间物种的形成证实,在25°C下,针状CaCO 3通过原位离子交换在Li 2 CO 3表面上异质形成。在60–100°C下,分离出的CaCO 3团聚是通过溶解-沉淀均匀形成的。温度升高加速了Li 2 CO 3的溶解和向LiOH的转化,从而产生了具有高[CO 3 2-- / [Ca 2+ ]比的溶液。这有利于分离出的CaCO 3附聚物的均匀沉淀。











































 京公网安备 11010802027423号
京公网安备 11010802027423号