当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coupled equilibria in H-bond donating ring-opening polymerization: The effective catalyst-determined shift of a polymerization equilibrium
European Polymer Journal ( IF 5.8 ) Pub Date : 2017-10-01 , DOI: 10.1016/j.eurpolymj.2017.05.018 Partha P. Datta , Jinal U. Pothupitiya , Elizabeth T. Kiesewetter , Matthew K. Kiesewetter
European Polymer Journal ( IF 5.8 ) Pub Date : 2017-10-01 , DOI: 10.1016/j.eurpolymj.2017.05.018 Partha P. Datta , Jinal U. Pothupitiya , Elizabeth T. Kiesewetter , Matthew K. Kiesewetter
|
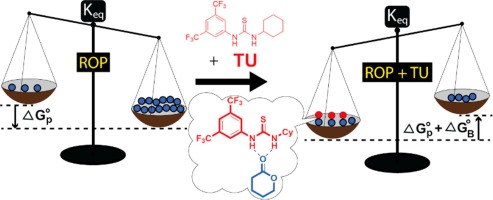
Abstract In the classic view of catalysis, a catalyst cannot alter the thermodynamically-determined endpoint of a reversible reaction. This conclusion is predicated on the assumption that the catalyst does not perturb the energy of product or reactant or does so to an equal extent. In the H-bond mediated ring-opening polymerization (ROP) of lactone monomers, the strength of the interactions of thiourea with product and reactant are not equal, and the magnitudes of these interactions are of similar energy to the free energy of reaction. The total monomer concentration at equilibrium in the thiourea/base cocatalyzed ROP of lactones is shown to be a function of the initial concentration of thiourea. Because the binding of thiourea to monomer and the polymerization reaction itself are both reversible, the application of varying amounts of thiourea catalyst directly alters the total amount of monomer in the reaction solution at equilibrium, which can be recovered at the end of the reaction.
中文翻译:

氢键供体开环聚合中的耦合平衡:聚合平衡的有效催化剂决定的转变
摘要 在催化的经典观点中,催化剂不能改变可逆反应的热力学终点。该结论基于催化剂不会干扰产物或反应物的能量或干扰程度相等的假设。在氢键介导的内酯单体开环聚合(ROP)中,硫脲与产物和反应物的相互作用强度不相等,并且这些相互作用的大小与反应的自由能具有相似的能量。在硫脲/碱共催化的内酯 ROP 中平衡时的总单体浓度显示为硫脲初始浓度的函数。因为硫脲与单体的结合和聚合反应本身都是可逆的,
更新日期:2017-10-01
中文翻译:

氢键供体开环聚合中的耦合平衡:聚合平衡的有效催化剂决定的转变
摘要 在催化的经典观点中,催化剂不能改变可逆反应的热力学终点。该结论基于催化剂不会干扰产物或反应物的能量或干扰程度相等的假设。在氢键介导的内酯单体开环聚合(ROP)中,硫脲与产物和反应物的相互作用强度不相等,并且这些相互作用的大小与反应的自由能具有相似的能量。在硫脲/碱共催化的内酯 ROP 中平衡时的总单体浓度显示为硫脲初始浓度的函数。因为硫脲与单体的结合和聚合反应本身都是可逆的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号