PLoS Pathogens ( IF 5.5 ) Pub Date : 2017-08-31 , DOI: 10.1371/journal.ppat.1006598 Dominik Hotter , Teresa Krabbe , Elisabeth Reith , Ali Gawanbacht , Nadia Rahm , Ahidjo Ayouba , Benoît Van Driessche , Carine Van Lint , Martine Peeters , Frank Kirchhoff , Daniel Sauter
|
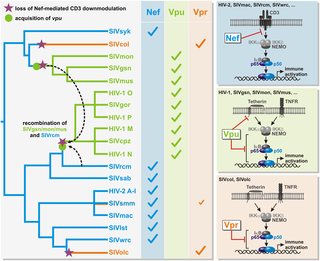
Primate lentiviruses have evolved sophisticated strategies to suppress the immune response of their host species. For example, HIV-2 and most simian immunodeficiency viruses (SIVs) use their accessory protein Nef to prevent T cell activation and antiviral gene expression by downmodulating the T cell receptor CD3. This Nef function was lost in HIV-1 and other vpu-encoding viruses suggesting that the acquisition of Vpu-mediated NF-κB inhibition reduced the selection pressure for inhibition of T cell activation by Nef. To obtain further insights into the modulation of NF-κB activity by primate lentiviral accessory factors, we analyzed 32 Vpr proteins from a large panel of divergent primate lentiviruses. We found that those of SIVcol and SIVolc infecting Colobinae monkeys showed the highest efficacy in suppressing NF-κB activation. Vpr-mediated inhibition of NF-κB resulted in decreased IFNβ promoter activity and suppressed type I IFN induction in virally infected primary cells. Interestingly, SIVcol and SIVolc differ from all other primate lentiviruses investigated by the lack of both, a vpu gene and efficient Nef-mediated downmodulation of CD3. Thus, primate lentiviruses have evolved at least three alternative strategies to inhibit NF-κB-dependent immune activation. Functional analyses showed that the inhibitory activity of SIVolc and SIVcol Vprs is independent of DCAF1 and the induction of cell cycle arrest. While both Vprs target the IKK complex or a factor further downstream in the NF-κB signaling cascade, only SIVolc Vpr stabilizes IκBα and inhibits p65 phosphorylation. Notably, only de-novo synthesized but not virion-associated Vpr suppressed the activation of NF-κB, thus enabling NF-κB-dependent initiation of viral gene transcription during early stages of the replication cycle, while minimizing antiviral gene expression at later stages. Our findings highlight the key role of NF-κB in antiviral immunity and demonstrate that primate lentiviruses follow distinct evolutionary paths to modulate NF-κB-dependent expression of viral and antiviral genes.
中文翻译:

灵长类慢病毒使用至少三种替代策略来抑制NF-κB介导的免疫激活
灵长类慢病毒已经发展出复杂的策略来抑制其宿主物种的免疫反应。例如,HIV-2和大多数猿猴免疫缺陷病毒(SIV)使用其辅助蛋白Nef通过下调T细胞受体CD3来防止T细胞活化和抗病毒基因表达。Nef功能在HIV-1和其他编码vpu的病毒中丢失,表明获得Vpu介导的NF-κB抑制作用降低了抑制Nef激活T细胞的选择压力。为了进一步了解灵长类慢病毒辅助因子对NF-κB活性的调节,我们分析了一大批不同的灵长类慢病毒中的32种Vpr蛋白。我们发现SIVcol和SIVolc感染了Colobinae猴子在抑制NF-κB活化方面显示出最高的功效。在病毒感染的原代细胞中,Vpr介导的NF-κB抑制导致IFNβ启动子活性降低和I型IFN诱导抑制。有趣的是,SIVcol和SIVolc与所有其他灵长类慢病毒有所不同,这两种病毒均缺乏vpu基因和有效的Nef介导的CD3下调。因此,灵长类慢病毒已经进化出至少三种抑制NF-κB依赖性免疫激活的替代策略。功能分析表明,SIVolc和SIVcol Vprs的抑制活性与DCAF1和细胞周期停滞的诱导无关。尽管两个Vpr都靶向IKK复合物或NF-κB信号级联反应下游的一个因子,但只有SIVolc Vpr可以稳定IκBα并抑制p65磷酸化。值得注意的是,只有从头合成但不与病毒体相关的Vpr抑制了NF-κB的激活,从而使NF-κB依赖性的病毒基因转录在复制周期的早期启动,而在后期将抗病毒基因的表达降至最低。我们的发现突出了NF-κB在抗病毒免疫中的关键作用,并证明灵长类慢病毒遵循不同的进化途径来调节病毒和抗病毒基因的NF-κB依赖性表达。









































 京公网安备 11010802027423号
京公网安备 11010802027423号