当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Functionalization of Azepane via Azomethine Ylides: A Highly Efficient Synthesis of Spirooxindoles Bearing a 1‐Azabicyclo[5.3.0]decane Moiety
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-09-07 , DOI: 10.1002/ajoc.201700384 Xiangtai Meng 1 , Yanlong Du 1 , Qi Zhang 1 , Aimin Yu 1 , Youquan Zhang 1 , Jiru Jia 1 , Xiujie Liu 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-09-07 , DOI: 10.1002/ajoc.201700384 Xiangtai Meng 1 , Yanlong Du 1 , Qi Zhang 1 , Aimin Yu 1 , Youquan Zhang 1 , Jiru Jia 1 , Xiujie Liu 1
Affiliation
|
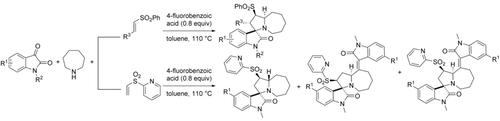
Spirooxindoles bearing a 1‐azabicyclo[5.3.0]decane moiety were synthesized in one step via direct functionalization of the azepane without a transition metal or oxidants. This is an efficient process for the synthesis of fused 7/5‐heterobicyclic systems in one step. Furthermore, cycloaddition of the in‐situ‐generated azomethine ylide only proceeded for alkenyl sulfone dipolarophiles.
中文翻译:

氮杂甲叉基叶立德直接将氮杂庚烷官能化:高效合成具有1-氮杂双环[5.3.0]癸烷部分的螺氧杂吲哚
含1-氮杂双环[5.3.0]癸烷部分的螺氧杂吲哚是在没有过渡金属或氧化剂的情况下通过氮杂环丙烷的直接官能化一步合成的。这是一步合成融合的7 / 5-杂环双环系统的有效方法。此外,原位生成的偶氮甲碱叶立德的环加成仅对烯基砜双极性亲和剂进行。
更新日期:2017-09-07
中文翻译:

氮杂甲叉基叶立德直接将氮杂庚烷官能化:高效合成具有1-氮杂双环[5.3.0]癸烷部分的螺氧杂吲哚
含1-氮杂双环[5.3.0]癸烷部分的螺氧杂吲哚是在没有过渡金属或氧化剂的情况下通过氮杂环丙烷的直接官能化一步合成的。这是一步合成融合的7 / 5-杂环双环系统的有效方法。此外,原位生成的偶氮甲碱叶立德的环加成仅对烯基砜双极性亲和剂进行。









































 京公网安备 11010802027423号
京公网安备 11010802027423号