当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu(TFA)2‐Catalyzed Picolinamido‐Directed C(sp2)−H Cyanation of Naphthalenes by Using Benzoyl Cyanide as a Cyano Source
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-08-31 , DOI: 10.1002/ajoc.201700418 He Song 1, 2 , Xiaochong Liu 1, 2 , Chenglong Wang 1, 2 , Jingyi Qiao 1, 2 , Wenyi Chu 1, 2 , Zhizhong Sun 1, 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-08-31 , DOI: 10.1002/ajoc.201700418 He Song 1, 2 , Xiaochong Liu 1, 2 , Chenglong Wang 1, 2 , Jingyi Qiao 1, 2 , Wenyi Chu 1, 2 , Zhizhong Sun 1, 2
Affiliation
|
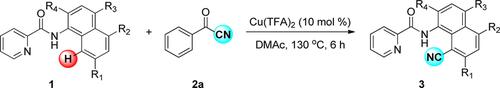
A protocol for the Cu(TFA)2‐catalyzed (TFA=trifluoroacetic acid) picolinamido‐directed C8−H cyanation of naphthalene derivatives with benzoyl cyanide as the cyano source has been developed. A series of 8‐cyano‐1‐(picolinamido)naphthalene derivatives were efficiently obtained in moderate to good yields by using this method. We prepared a total of 22 products, 10 of which have not previously been reported. Benzoyl cyanide was originally employed for the C(sp2)−H cyanation of arenes. The picolinamide moiety served a critical role as the directing group in this cyanation reaction of naphthalenes.
中文翻译:

Cu(TFA)2-催化苯甲酰氰化物为氰源的萘类的吡咯并氨基指示的C(sp2)-H氰化反应
已开发出一种以铜(TFA)2催化(TFA =三氟乙酸)吡啶衍生物指导的萘衍生物以苯甲酰氰为氰基氰化C8-H氰化的方案。使用此方法可以有效地以中等到良好的产率获得一系列8-氰基-1-(吡啶并氨基)萘衍生物。我们总共准备了22种产品,其中10种以前没有报道过。苯甲酰氰最初用于芳烃的C(sp 2)-H氰化。吡啶甲酰胺部分在萘的氰化反应中起着指导基团的作用。
更新日期:2017-08-31
中文翻译:

Cu(TFA)2-催化苯甲酰氰化物为氰源的萘类的吡咯并氨基指示的C(sp2)-H氰化反应
已开发出一种以铜(TFA)2催化(TFA =三氟乙酸)吡啶衍生物指导的萘衍生物以苯甲酰氰为氰基氰化C8-H氰化的方案。使用此方法可以有效地以中等到良好的产率获得一系列8-氰基-1-(吡啶并氨基)萘衍生物。我们总共准备了22种产品,其中10种以前没有报道过。苯甲酰氰最初用于芳烃的C(sp 2)-H氰化。吡啶甲酰胺部分在萘的氰化反应中起着指导基团的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号