当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Synthesis of Silacycles by Borane-Catalyzed Domino Hydrosilylation of Proximal Unsaturated Bonds: Tunable Approach to 1,n-Diols
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-09-13 04:30:51 , DOI: 10.1002/adsc.201700698 Kwangmin Shin 1, 2 , Seewon Joung 1, 2 , Youyoung Kim 1, 2 , Sukbok Chang 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-09-13 04:30:51 , DOI: 10.1002/adsc.201700698 Kwangmin Shin 1, 2 , Seewon Joung 1, 2 , Youyoung Kim 1, 2 , Sukbok Chang 1, 2
Affiliation
|
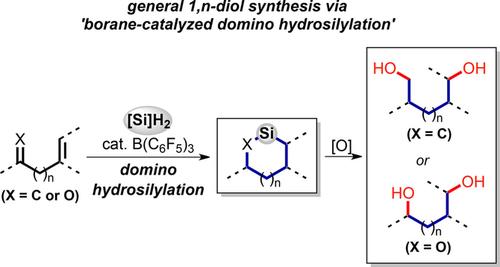
The tris(pentafluorophenyl)boron-catalyzed domino hydrosilylation of substrates carrying unsaturated functionalities in a proximal arrangement is presented to produce silacycles. Excellent levels of efficiency and selectivity were achieved in the cyclization by the deliberate choice of the hydrosilane reagents. The key to successful cyclic hydrosilylation is the reactivity enhancement of the second intramolecular hydrosilylation by a proximity effect. Not only dienes but also enones, enynes, ynones and enimines readily afford medium-sized silacycles under convenient and mild conditions. The cyclization proceeds with acceptable diastereoselectivity mainly controlled by the conformational bias towards inducing additional stereogenic centers. The silacycles obtained from this reaction were converted to 1,n-diols or 1,n-amino alcohols upon oxidation, thus rendering the present cyclization a powerful tool for accessing synthetically valuable building blocks.
中文翻译:

通过硼烷催化的不饱和键的多米诺加氢硅烷化选择性合成硅环的方法:1,n-二元醇的可调方法
提出了在近端排列中携带不饱和官能团的底物的三(五氟苯基)硼催化的多米诺骨硅氢化反应,以产生silacycles。通过精心选择氢化硅烷试剂,可以在环化反应中获得出色的效率和选择性。成功的环状氢化硅烷化的关键是通过邻近效应提高第二次分子内氢化硅烷化的反应性。在方便和温和的条件下,不仅二烯而且烯酮,烯炔,炔酮和亚胺都容易得到中等大小的硅杂环。环化以可接受的非对映选择性进行,该非对映选择性主要受构象偏向诱导额外的立体异构中心的控制。从该反应获得的硅环在氧化后被转化为1,n-二醇或1,n-氨基醇。
更新日期:2017-09-13
中文翻译:

通过硼烷催化的不饱和键的多米诺加氢硅烷化选择性合成硅环的方法:1,n-二元醇的可调方法
提出了在近端排列中携带不饱和官能团的底物的三(五氟苯基)硼催化的多米诺骨硅氢化反应,以产生silacycles。通过精心选择氢化硅烷试剂,可以在环化反应中获得出色的效率和选择性。成功的环状氢化硅烷化的关键是通过邻近效应提高第二次分子内氢化硅烷化的反应性。在方便和温和的条件下,不仅二烯而且烯酮,烯炔,炔酮和亚胺都容易得到中等大小的硅杂环。环化以可接受的非对映选择性进行,该非对映选择性主要受构象偏向诱导额外的立体异构中心的控制。从该反应获得的硅环在氧化后被转化为1,n-二醇或1,n-氨基醇。









































 京公网安备 11010802027423号
京公网安备 11010802027423号