当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DNA-Compatible Nitro Reduction and Synthesis of Benzimidazoles
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00416 Huang-Chi Du 1 , Hongbing Huang 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00416 Huang-Chi Du 1 , Hongbing Huang 1
Affiliation
|
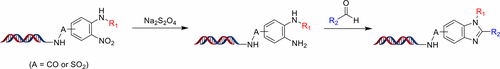
DNA-encoded chemical libraries have emerged as a cost-effective alternative to high-throughput screening (HTS) for hit identification in drug discovery. A key factor for productive DNA-encoded libraries is the chemical diversity of the small molecule moiety attached to an encoding DNA oligomer. The library structure diversity is often limited to DNA-compatible chemical reactions in aqueous media. Herein, we describe a facile process for reducing aryl nitro groups to aryl amines. The new protocol offers simple operation and circumvents the pyrophoric potential of the conventional method (Raney nickel). The reaction is performed in aqueous solution and does not compromise DNA structural integrity. The utility of this method is demonstrated by the versatile synthesis of benzimidazoles on DNA.
中文翻译:

DNA兼容的硝基还原和苯并咪唑的合成
DNA编码的化学文库已经成为高通量筛选(HTS)的一种经济有效的替代方法,用于在药物发现中进行命中鉴定。高效的DNA编码文库的关键因素是连接至编码DNA寡聚物的小分子部分的化学多样性。文库结构的多样性通常仅限于水性介质中与DNA相容的化学反应。在本文中,我们描述了将芳基硝基还原为芳基胺的简便方法。新协议提供了简单的操作,并规避了常规方法(阮内镍)的自燃性。该反应在水溶液中进行,不会损害DNA结构的完整性。苯并咪唑在DNA上的多功能合成证明了该方法的实用性。
更新日期:2017-09-13
中文翻译:

DNA兼容的硝基还原和苯并咪唑的合成
DNA编码的化学文库已经成为高通量筛选(HTS)的一种经济有效的替代方法,用于在药物发现中进行命中鉴定。高效的DNA编码文库的关键因素是连接至编码DNA寡聚物的小分子部分的化学多样性。文库结构的多样性通常仅限于水性介质中与DNA相容的化学反应。在本文中,我们描述了将芳基硝基还原为芳基胺的简便方法。新协议提供了简单的操作,并规避了常规方法(阮内镍)的自燃性。该反应在水溶液中进行,不会损害DNA结构的完整性。苯并咪唑在DNA上的多功能合成证明了该方法的实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号