当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein–polysaccharide complexes for enhanced protein delivery in hyaluronic acid templated calcium carbonate microparticles
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2017-08-16 00:00:00 , DOI: 10.1039/c7tb01538k Bathabile Ramalapa 1, 2, 3, 4, 5 , Oscar Crasson 6, 7, 8, 9, 10 , Marylène Vandevenne 6, 7, 8, 9, 10 , Alain Gibaud 11, 12, 13 , Emmanuel Garcion 1, 2, 3, 4, 5 , Thomas Cordonnier 1, 2, 3, 4, 5 , Moreno Galleni 6, 7, 8, 9, 10 , Frank Boury 1, 2, 3, 4, 5
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2017-08-16 00:00:00 , DOI: 10.1039/c7tb01538k Bathabile Ramalapa 1, 2, 3, 4, 5 , Oscar Crasson 6, 7, 8, 9, 10 , Marylène Vandevenne 6, 7, 8, 9, 10 , Alain Gibaud 11, 12, 13 , Emmanuel Garcion 1, 2, 3, 4, 5 , Thomas Cordonnier 1, 2, 3, 4, 5 , Moreno Galleni 6, 7, 8, 9, 10 , Frank Boury 1, 2, 3, 4, 5
Affiliation
|
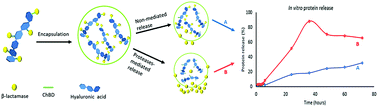
The controlled delivery of proteins within calcium carbonate (CaCO3) particles is currently widely investigated. The success of these carriers is driven by ionic interactions between the encapsulated proteins and the particles. This poses a great limitation on the successful loading of proteins that have no ionic affinity to CaCO3. In this study, we explored the use of polysaccharide–protein interactions to strongly enhance the encapsulation of proteins in CaCO3 microparticles. Previously, Vandevenne and colleagues inserted a human chitin binding domain (ChBD) that has intrinsic affinity for hyaluronic acid (HA) into a β-lactamase (BlaP). This generated chimeric protein, named BlaPChBD, was shown to be fully bifunctional. In this study we showed that this hybrid protein can associate with HA and be successfully loaded into vaterite CaCO3 microparticles using supercritical CO2 (ScCO2) technology aided by the templating effect of HA on CaCO3. The presence of ChBD inserted into BlaP increased the encapsulation of the protein by 6-fold when complexed with HA. Furthermore, thrombin cleavage sites were engineered on both sides of the inserted ChBD in the chimeric BlaP to achieve release of the protein from the microparticles by protease cleavage. Our results showed that thrombin cleavage increased the release of the protein from the microparticles within 36 hours from <20% to 87%. In conclusion, the presence of ChBD successfully improved the encapsulation yield of the protein while retaining up to 82% of its activity and efficient release of the protein from the microparticles was achieved by protease cleavage.
中文翻译:

蛋白-多糖复合物可增强透明质酸模板碳酸钙微粒中的蛋白质传递
目前,人们广泛研究了碳酸钙(CaCO 3)颗粒内蛋白质的受控递送。这些载体的成功是由包封的蛋白质和颗粒之间的离子相互作用驱动的。这对成功装载对CaCO 3没有离子亲和力的蛋白质构成了很大的限制。在这项研究中,我们探索了使用多糖与蛋白质的相互作用来强烈增强蛋白质在CaCO 3中的包裹微粒。以前,Vandevenne及其同事将对透明质酸(HA)具有内在亲和力的人几丁质结合域(ChBD)插入到β-内酰胺酶(BlaP)中。这种产生的称为BlaPChBD的嵌合蛋白被证明是完全双功能的。在这项研究中,我们证明了这种杂合蛋白可以与HA结合,并借助HA对CaCO 3的模板作用,利用超临界CO 2(ScCO 2)技术成功地将其装载到球ate石CaCO 3微粒中。。当与HA复合时,插入BlaP的ChBD的存在使蛋白质的封装增加了6倍。此外,在嵌合的BlaP中插入的ChBD的两侧工程改造了凝血酶切割位点,以通过蛋白酶切割实现蛋白质从微粒中的释放。我们的结果表明,凝血酶裂解可在36小时内将蛋白质从微粒中的释放从<20%增加到87%。总之,ChBD的存在成功提高了蛋白质的包封率,同时保留了高达82%的活性,并且通过蛋白酶裂解实现了蛋白质从微粒中的有效释放。
更新日期:2017-09-13
中文翻译:

蛋白-多糖复合物可增强透明质酸模板碳酸钙微粒中的蛋白质传递
目前,人们广泛研究了碳酸钙(CaCO 3)颗粒内蛋白质的受控递送。这些载体的成功是由包封的蛋白质和颗粒之间的离子相互作用驱动的。这对成功装载对CaCO 3没有离子亲和力的蛋白质构成了很大的限制。在这项研究中,我们探索了使用多糖与蛋白质的相互作用来强烈增强蛋白质在CaCO 3中的包裹微粒。以前,Vandevenne及其同事将对透明质酸(HA)具有内在亲和力的人几丁质结合域(ChBD)插入到β-内酰胺酶(BlaP)中。这种产生的称为BlaPChBD的嵌合蛋白被证明是完全双功能的。在这项研究中,我们证明了这种杂合蛋白可以与HA结合,并借助HA对CaCO 3的模板作用,利用超临界CO 2(ScCO 2)技术成功地将其装载到球ate石CaCO 3微粒中。。当与HA复合时,插入BlaP的ChBD的存在使蛋白质的封装增加了6倍。此外,在嵌合的BlaP中插入的ChBD的两侧工程改造了凝血酶切割位点,以通过蛋白酶切割实现蛋白质从微粒中的释放。我们的结果表明,凝血酶裂解可在36小时内将蛋白质从微粒中的释放从<20%增加到87%。总之,ChBD的存在成功提高了蛋白质的包封率,同时保留了高达82%的活性,并且通过蛋白酶裂解实现了蛋白质从微粒中的有效释放。











































 京公网安备 11010802027423号
京公网安备 11010802027423号