当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evaporation of liquid droplets of nano- and micro-meter size as a function of molecular mass and intermolecular interactions: experiments and molecular dynamics simulations
Soft Matter ( IF 3.4 ) Pub Date : 2017-07-26 00:00:00 , DOI: 10.1039/c7sm00804j Robert Hołyst 1, 2, 3 , Marek Litniewski 1, 2, 3 , Daniel Jakubczyk 3, 4, 5
Soft Matter ( IF 3.4 ) Pub Date : 2017-07-26 00:00:00 , DOI: 10.1039/c7sm00804j Robert Hołyst 1, 2, 3 , Marek Litniewski 1, 2, 3 , Daniel Jakubczyk 3, 4, 5
Affiliation
|
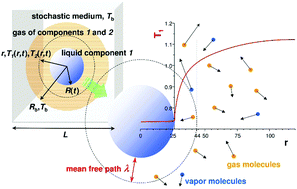
Transport of heat to the surface of a liquid is a limiting step in the evaporation of liquids into an inert gas. Molecular dynamics (MD) simulations of a two component Lennard-Jones (LJ) fluid revealed two modes of energy transport from a vapour to an interface of an evaporating droplet of liquid. Heat is transported according to the equation of temperature diffusion, far from the droplet of radius R. The heat flux, in this region, is proportional to temperature gradient and heat conductivity in the vapour. However at some distance from the interface, Aλ, (where λ is the mean free path in the gas), the temperature has a discontinuity and heat is transported ballistically i.e. by direct individual collisions of gas molecules with the interface. This ballistic transport reduces the heat flux (and consequently the mass flux) by the factor R/(R + Aλ) in comparison to the flux obtained from temperature diffusion. Thus it slows down the evaporation of droplets of sizes R ∼ Aλ and smaller (practically for sizes from 103 nm down to 1 nm). We analyzed parameter A as a function of interactions between molecules and their masses. The rescaled parameter, A(kBTb/ε11)1/2, is a linear function of the ratio of the molecular mass of the liquid molecules to the molecular mass of the gas molecules, m1/m2 (for a series of chemically similar compounds). Here ε11 is the interaction parameter between molecules in the liquid (proportional to the enthalpy of evaporation) and Tb is the temperature of the gas in the bulk. We tested the predictions of MD simulations in experiments performed on droplets of ethylene glycol, diethylene glycol, triethylene glycol and tetraethylene glycol. They were suspended in an electrodynamic trap and evaporated into dry nitrogen gas. A changes from ∼1 (for ethylene glycol) to approximately 10 (for tetraethylene glycol) and has the same dependence on molecular parameters as obtained for the LJ fluid in MD simulations. The value of x = A(kBTb/ε11)1/2 is of the order of 1 (for water x = 1.8, glycerol x = 1, ethylene glycol x = 0.4, tetraethylene glycol x = 2.1 evaporating into dry nitrogen at room temperature and for Lennard-Jones fluids x = 2 for m1/m2 = 1 and low temperature).
中文翻译:

纳米和微米大小液滴的蒸发与分子质量和分子间相互作用的关系:实验和分子动力学模拟
将热量传输到液体表面是将液体蒸发成惰性气体的限制步骤。两组分Lennard-Jones(LJ)流体的分子动力学(MD)模拟显示了从蒸汽到液体蒸发液滴界面的两种能量传输模式。根据温度扩散方程,热量被传输到远离半径为R的液滴的位置。在该区域中,热通量与蒸气中的温度梯度和热导率成正比。但是,在距界面Aλ一定距离处(其中λ是气体中的平均自由程),温度具有不连续性,并且热量以弹道方式传输,即气体分子与界面的直接单个碰撞。与从温度扩散获得的热通量相比,这种弹道传输将热通量(以及质量通量)减少了R /(R + Aλ)倍。因此,它减慢尺寸的液滴的蒸发- [R 〜Aλ和更小(几乎从10尺寸3纳米到1纳米)。我们分析了作为分子与其质量之间相互作用的函数的参数A。经重新缩放参数,甲(ķ乙Ť b / ε 11)1/2,是液体分子的分子量与气体分子的分子量之比m 1 / m 2(对于一系列化学相似的化合物)的线性函数。这里ε 11是在液体分子之间的相互作用参数(正比于蒸发焓)和Ť b是气体在本体的温度。我们在乙二醇,二甘醇,三甘醇和四甘醇的液滴上进行的实验中测试了MD模拟的预测。将它们悬浮在电动捕集阱中,并蒸发成干燥的氮气。一个变化从〜1(对于乙二醇)到大约10(对于四乙二醇),并且对分子参数的依赖性与在MD模拟中对LJ流体获得的依赖性相同。的值X =甲(ķ乙Ť b / ε 11)1/2是1的量级(对于水X = 1.8,甘油X = 1,乙二醇X = 0.4,四甘醇X = 2.1蒸发到干对于m 1 / m 2 = 1和低温,在室温下和Lennard-Jones流体x = 2时为氮气。
更新日期:2017-09-13
中文翻译:

纳米和微米大小液滴的蒸发与分子质量和分子间相互作用的关系:实验和分子动力学模拟
将热量传输到液体表面是将液体蒸发成惰性气体的限制步骤。两组分Lennard-Jones(LJ)流体的分子动力学(MD)模拟显示了从蒸汽到液体蒸发液滴界面的两种能量传输模式。根据温度扩散方程,热量被传输到远离半径为R的液滴的位置。在该区域中,热通量与蒸气中的温度梯度和热导率成正比。但是,在距界面Aλ一定距离处(其中λ是气体中的平均自由程),温度具有不连续性,并且热量以弹道方式传输,即气体分子与界面的直接单个碰撞。与从温度扩散获得的热通量相比,这种弹道传输将热通量(以及质量通量)减少了R /(R + Aλ)倍。因此,它减慢尺寸的液滴的蒸发- [R 〜Aλ和更小(几乎从10尺寸3纳米到1纳米)。我们分析了作为分子与其质量之间相互作用的函数的参数A。经重新缩放参数,甲(ķ乙Ť b / ε 11)1/2,是液体分子的分子量与气体分子的分子量之比m 1 / m 2(对于一系列化学相似的化合物)的线性函数。这里ε 11是在液体分子之间的相互作用参数(正比于蒸发焓)和Ť b是气体在本体的温度。我们在乙二醇,二甘醇,三甘醇和四甘醇的液滴上进行的实验中测试了MD模拟的预测。将它们悬浮在电动捕集阱中,并蒸发成干燥的氮气。一个变化从〜1(对于乙二醇)到大约10(对于四乙二醇),并且对分子参数的依赖性与在MD模拟中对LJ流体获得的依赖性相同。的值X =甲(ķ乙Ť b / ε 11)1/2是1的量级(对于水X = 1.8,甘油X = 1,乙二醇X = 0.4,四甘醇X = 2.1蒸发到干对于m 1 / m 2 = 1和低温,在室温下和Lennard-Jones流体x = 2时为氮气。



























 京公网安备 11010802027423号
京公网安备 11010802027423号