当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Reaction Mechanism of Criegee Intermediate CH2OO with Methane and Implications for the Formation of Methanol
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.jpca.7b05858 Kaining Xu 1 , Weihua Wang 1 , Wenjing Wei 1 , Wenling Feng 1 , Qiao Sun 2 , Ping Li 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.jpca.7b05858 Kaining Xu 1 , Weihua Wang 1 , Wenjing Wei 1 , Wenling Feng 1 , Qiao Sun 2 , Ping Li 1
Affiliation
|
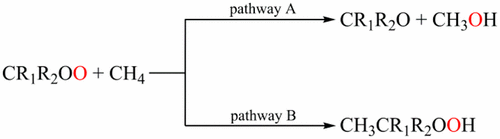
Criegee intermediates (CIs) play a key role in controlling the atmospheric budget of hydroxyl radical, organic acids, and secondary organic aerosols. In this study, the detailed reaction mechanisms of the simplest Criegee intermediate CH2OO and its derivatives with methane (CH4) have been systematically investigated theoretically. Two pathways A and B have been identified for the title reaction. In pathway A, CIs can act as an oxygen donor by inserting its terminal oxygen atom into the C–H bond of alkanes, resulting in the formation of alcohol species. The corresponding energy barriers ranging from 6.5 to 24.1 kcal/mol are associated with the O–O bond strength of CIs. Meanwhile, this pathway is more favorable thermodynamically, where the free energy changes (enthalpy changes) range from −81.1 (−78.3) to −110.9 (−109.0) kcal/mol, respectively. In pathway B, an addition reaction to produce the hydroperoxides occurs, accompanying the hydrogen transfer from the alkanes to the terminal oxygen atom of CIs. The corresponding energy barriers ranging from 17.3 to 30.9 kcal/mol are higher than those in pathway A. Further calculations of the rate constants suggest that pathway A is the most favorable reaction channel and the rate constant exhibits a positive temperature dependence. In addition, the conformation-dependent reactivity for the title reaction has been observed. The present findings can enable us to better understand the potential reactivity of CIs in the presence of the alkane species.
中文翻译:

Criegee中间体CH 2 OO与甲烷的反应机理及对甲醇形成的启示
Criegee中间体(CIs)在控制大气中的羟基自由基,有机酸和次要有机气溶胶方面起着关键作用。在这项研究中,最简单的Criegee中间体CH 2 OO及其衍生物与甲烷(CH 4)已从理论上进行了系统的研究。已经为标题反应确定了两条途径A和B。在途径A中,CI可以通过将其末端的氧原子插入烷烃的C–H键来充当供氧体,从而导致形成醇类。相应的6.5至24.1 kcal / mol的能垒与CI的O-O键强度有关。同时,该途径在热力学上更有利,其自由能变化(焓变)分别为-81.1(-78.3)至-110.9(-109.0)kcal / mol。在途径B中,伴随着氢从烷烃向CIs末端氧原子的转移,发生了产生氢过氧化物的加成反应。相应的能垒范围为17.3至30.9 kcal / mol,高于途径A中的能垒。速率常数的进一步计算表明,途径A是最有利的反应通道,速率常数表现出正的温度依赖性。另外,已经观察到标题反应的构象依赖性反应性。目前的发现可以使我们更好地理解在烷烃存在下CIs的潜在反应性。
更新日期:2017-09-13
中文翻译:

Criegee中间体CH 2 OO与甲烷的反应机理及对甲醇形成的启示
Criegee中间体(CIs)在控制大气中的羟基自由基,有机酸和次要有机气溶胶方面起着关键作用。在这项研究中,最简单的Criegee中间体CH 2 OO及其衍生物与甲烷(CH 4)已从理论上进行了系统的研究。已经为标题反应确定了两条途径A和B。在途径A中,CI可以通过将其末端的氧原子插入烷烃的C–H键来充当供氧体,从而导致形成醇类。相应的6.5至24.1 kcal / mol的能垒与CI的O-O键强度有关。同时,该途径在热力学上更有利,其自由能变化(焓变)分别为-81.1(-78.3)至-110.9(-109.0)kcal / mol。在途径B中,伴随着氢从烷烃向CIs末端氧原子的转移,发生了产生氢过氧化物的加成反应。相应的能垒范围为17.3至30.9 kcal / mol,高于途径A中的能垒。速率常数的进一步计算表明,途径A是最有利的反应通道,速率常数表现出正的温度依赖性。另外,已经观察到标题反应的构象依赖性反应性。目前的发现可以使我们更好地理解在烷烃存在下CIs的潜在反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号