当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Study and Modeling of the UV–Vis and Infrared Spectra of the [VO(O2)Hheida]− Complex Dissolved in Water
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.jpca.7b07128 S. Klokishner 1 , O. Reu 1 , J. Noack 2 , R. Schlögl 2 , A. Trunschke 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.jpca.7b07128 S. Klokishner 1 , O. Reu 1 , J. Noack 2 , R. Schlögl 2 , A. Trunschke 2
Affiliation
|
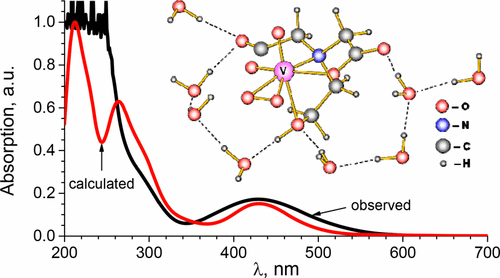
Combined theoretical and experimental studies of the [VO(O2)Hheida]− anion dissolved in water that may serve as a functional model for vanadium haloperoxidase enzymes have been performed. The geometrical structure and absorption and vibrational spectra of this system have been evaluated within the framework of density functional theory (DFT). The obtained theoretical results on the equilibrium structure and optical spectra are in quite good agreement with the experimental data. With the aid of the combination of UV–visible spectroscopy and electronic structure calculations, it has been revealed that, in the apparent absorption spectra of the [VO(O2)Hheida]− anion, the highest in energy band corresponds to a ligand to metal electron excitation, while the band with a maximum at 430 nm arises from the peroxo group. The calculations also reproduce quite well the positions, intensities and the grouping of frequencies in the near-infrared (NIR) spectra. The visualization of the calculated vibrations in the energy range of 400–1100 cm–1 has been presented.
中文翻译:

[VO(O 2)Hheida] -溶于水的UV-Vis和红外光谱的实验研究和建模
结合了[VO(O 2)Hheida] -阴离子溶解在水中的理论和实验研究,该阴离子可作为钒卤代过氧化物酶的功能模型。该系统的几何结构,吸收和振动光谱已在密度泛函理论(DFT)的框架内进行了评估。所获得的关于平衡结构和光谱的理论结果与实验数据非常吻合。借助于紫外可见光谱法和电子结构计算的组合,已经发现,在[VO(O 2)Hheida]的表观吸收光谱中-阴离子的能带最高对应于金属电子激发的配体,而在430 nm处最大的能带则来自于过氧基团。计算结果也很好地再现了近红外(NIR)光谱中的位置,强度和频率分组。给出了在400–1100 cm –1能量范围内计算出的振动的可视化。
更新日期:2017-09-13
中文翻译:

[VO(O 2)Hheida] -溶于水的UV-Vis和红外光谱的实验研究和建模
结合了[VO(O 2)Hheida] -阴离子溶解在水中的理论和实验研究,该阴离子可作为钒卤代过氧化物酶的功能模型。该系统的几何结构,吸收和振动光谱已在密度泛函理论(DFT)的框架内进行了评估。所获得的关于平衡结构和光谱的理论结果与实验数据非常吻合。借助于紫外可见光谱法和电子结构计算的组合,已经发现,在[VO(O 2)Hheida]的表观吸收光谱中-阴离子的能带最高对应于金属电子激发的配体,而在430 nm处最大的能带则来自于过氧基团。计算结果也很好地再现了近红外(NIR)光谱中的位置,强度和频率分组。给出了在400–1100 cm –1能量范围内计算出的振动的可视化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号