当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Synthesis of Stereochemically Defined Enamides via Bi- and Tricomponent Coupling Reaction
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.orglett.7b02166 Y. Liu 1 , A. De Nisi 1 , A. Cerveri 1 , M. Monari 1 , M. Bandini 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.orglett.7b02166 Y. Liu 1 , A. De Nisi 1 , A. Cerveri 1 , M. Monari 1 , M. Bandini 1
Affiliation
|
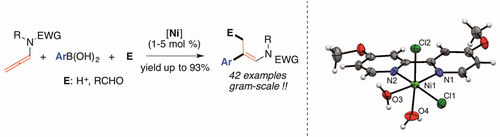
The stereoselective synthesis of (E)-trisubstituted tertiary enamides is documented via site-selective Ni-catalyzed β-arylation of allenamides with boronic acids in high yields (up to 89%). The nucleophilic character of the “organo-Ni” intermediates is further exploited to implement a one-pot tricomponent procedure involving the final allylation of aldehydes (yields up to 93%). Mechanistic insights and efficiency on a gram scale process were also documented.
中文翻译:

镍催化的双组分和三组分偶联反应合成立体化学定义的酰胺
(E)-三取代叔烯酰胺的立体选择性合成是通过高选择性(最高89%)的烯丙基酰胺与硼酸的位点选择性Ni催化的β-芳基化进行记录的。进一步利用“有机镍”中间体的亲核特性来实施一锅三组分方法,该方法涉及醛的最终烯丙基化(收率高达93%)。还记录了克级过程的机械原理和效率。
更新日期:2017-09-13
中文翻译:

镍催化的双组分和三组分偶联反应合成立体化学定义的酰胺
(E)-三取代叔烯酰胺的立体选择性合成是通过高选择性(最高89%)的烯丙基酰胺与硼酸的位点选择性Ni催化的β-芳基化进行记录的。进一步利用“有机镍”中间体的亲核特性来实施一锅三组分方法,该方法涉及醛的最终烯丙基化(收率高达93%)。还记录了克级过程的机械原理和效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号