当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Making Flavone Thioethers Using Halides and Powdered Sulfur or Na2S2O3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.joc.7b01320 Qiujie Tang 1 , Zhaogang Bian 1 , Wei Wu 1 , Jin Wang 1 , Ping Xie 2 , Charles U. Pittman 3 , Aihua Zhou 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.joc.7b01320 Qiujie Tang 1 , Zhaogang Bian 1 , Wei Wu 1 , Jin Wang 1 , Ping Xie 2 , Charles U. Pittman 3 , Aihua Zhou 1
Affiliation
|
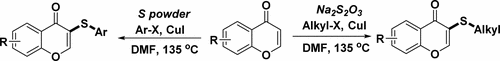
The method for constructing C–S bonds is very important in organic synthesis. Here a new sulfenylation method to generate flavone thioether derivatives was developed by employing aromatic or alkyl halides, S powder and Na2S2O3 as reactants. Good yields of regioselective Calkyl–S and Caryl–S-substituted flavones were generated under relatively environmentally friendly and simple conditions. This method might be potentially applicable to large scale production, and it enriches current sulfenylation methods.
中文翻译:

使用卤化物和粉状硫或Na 2 S 2 O 3制备黄酮硫醚
构造CS键的方法在有机合成中非常重要。在这里,通过使用芳族或烷基卤化物,S粉和Na 2 S 2 O 3作为反应物,开发了一种新的磺酰化方法以生成黄酮硫醚衍生物。在相对环境友好和简单的条件下,可以产生高选择性的区域选择性C烷基-S和C芳基-S取代的黄酮。该方法可能潜在地适用于大规模生产,并且丰富了当前的亚磺酰化方法。
更新日期:2017-09-13
中文翻译:

使用卤化物和粉状硫或Na 2 S 2 O 3制备黄酮硫醚
构造CS键的方法在有机合成中非常重要。在这里,通过使用芳族或烷基卤化物,S粉和Na 2 S 2 O 3作为反应物,开发了一种新的磺酰化方法以生成黄酮硫醚衍生物。在相对环境友好和简单的条件下,可以产生高选择性的区域选择性C烷基-S和C芳基-S取代的黄酮。该方法可能潜在地适用于大规模生产,并且丰富了当前的亚磺酰化方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号